iSTAR Medical SA
- Home
- Companies
- iSTAR Medical SA
- Applications
- Micro-Invasive Glaucoma Surgery Device ...

Micro-Invasive Glaucoma Surgery Device (MIGS) for Clinical Trial Program Supports - Medical / Health Care - Clinical Services
FromiSTAR Medical SA
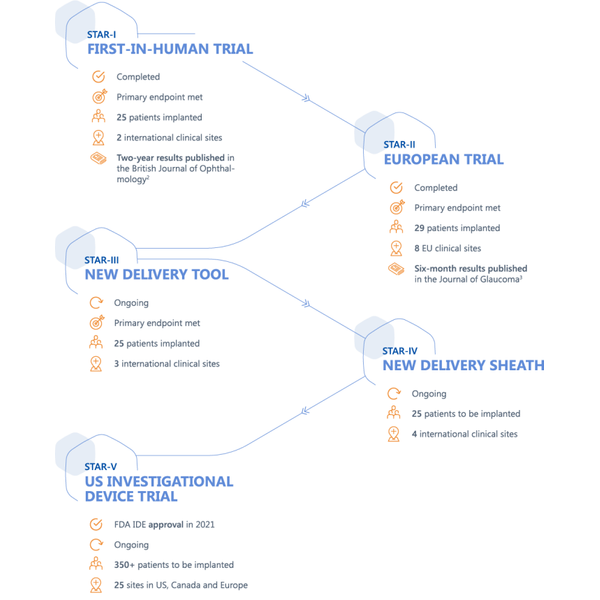
A comprehensive clinical trial program supports the development of MINIject®, with positive combined results at two years from the STAR-I and STAR-II clinical trials shown below. 1
Most popular related searches
clinical trial
intraocular pressure
clinical program
eye irritation
eye irritant
eye irritations
cataract surgery
skin sensitization
surgery
glaucoma surgery
Intraocular Pressure (IOP)
- Meaningful IOP reduction to
- ~40% mean IOP reduction after two years
- Trials designed with stand-alone procedure
Medication
- Nearly half of patients medication-free after two years
- Medication use reduced by 45% two years post-procedure
- Significant reduction or elimination of medication side-effects such as eye irritation/burning, eye pain, and skin sensitivity/irritation around the eye ²
Reduced complications
- Low re-intervention rate up to two years
- Bleb-free and conjunctiva sparing procedure - no needling, no MMC required
- Very low number of patients with >30% central endothelial cell density (ECD) loss at two years
Evidence-based data on safety and efficacy
- More than 150 patients in clinical trials
- 11 trial centers throughout Europe, India and Latin America
- MINIject assessed as stand-alone procedure- no enhancement of outcomes from joint cataract surgery
- Published results after two-years of follow-up