

- Home
- Companies
- Sanara MedTech Inc.
- Articles
- ACTIGEN Verified Inductive Bone Matrix ...
ACTIGEN Verified Inductive Bone Matrix and Autologous Blood by CellRight Technologies
Background
CellRight Technologies® has developed ACTIGEN™ Verified Inductive Bone Matrix, a dehydrated, verified inductive matrix that may be hydrated with saline, blood, Bone Marrow Aspirate (BMA), Platelet Rich Plasma (PRP), or other cellular components in accordance with a physician’s well-informed medical judgment. The clinician may add allograft or autograft to ACTIGEN™ Verified Inductive Bone Matrix and hydrate the grafting material to the desired consistency. ACTIGEN™ Verified Inductive Bone Matrix does not contain any extrinsic carriers and is entirely derived from 100% human allograft bone. ACTIGEN™ Verified Inductive Bone Matrix resists irrigation and has a shelf-life of 5 years from the date of internal packaging. CellRight Technologies® uses a proprietary demineralization process designed to preserve native BMP’s, as demonstrated in their osteoinductive testing. Every lot of ACTIGEN™ Verified Inductive Bone Matrix is verified for osteoinductivity post sterilization as a condition for distribution. In-vivo test results have histologically demonstrated the presence of all 5 bone-forming elements (Chondrocytes, Osteocytes, Bone Marrow Cells, Cartilage, and New Bone). ACTIGEN™ Verified Inductive Bone Matrix has also been in-vitro lot tested for the endogenous BMP-2 test marker for osteoinductivity. Studies show that BMP’s irreversibly induce differentiation of perivascular mesenchymal-type cells into osteoprogenitor cells.
It is well documented that not all DBM’s are created equal. How a DBM is processed and formulated have the biggest effects on its potential efficacy (1). There is a positive association between greater % DBM-base (bone powder) in the DBM-base product and higher fusion rate (2). Moreover, it doesn’t matter how great your cell is, if you don’t deliver it with the right matrix with appropriate cell-friendly
characteristics, cells will not likely survive after implantation (3).
A 5cc sample of ACTIGEN™ Verified Inductive Bone Matrix was provided to a well known U.S. cell-concentration device manufacturer for the purpose of rehydrating ACTIGEN™ Verified Inductive Bone Matrix with a fluid rich in growth factors (GFC) and mesenchymal stem cells (MSC’s). The study was designed to measure percent cell viability over one (1) hour as a worse case scenario. Current clinical practice is to implant the patient’s concentrated stem cells back into the patient as quickly as possible in an effort to maintain cell viability.
- Mix 0.5 cc of ACTIGENTM Verified Inductive Bone Matrix with 0.5 cc of fluid containing 250,000 MSC’s /GFC with a spatula, standard equipment available in the OR
- Incubate material at room temperature for 60 minutes
- At designated time points of 15, 30, and 60 minutes, gently rinse ACTIGENTM Verified Inductive Bone Matrix with 1cc of PBS (without Ca or Mg) so as to not disturb the material
- Using a nucleocounter, determine the number of unbound, living cells in the sample
- The percentage of bound cells was determined
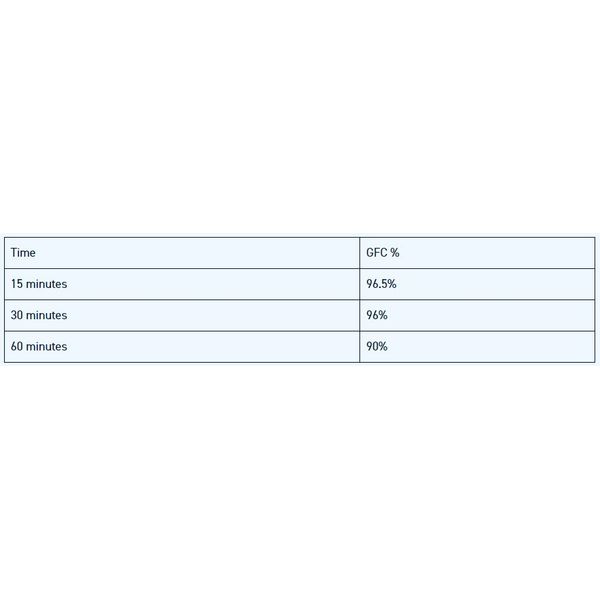
This investigation corroborates a previous independent study performed by a U.S. platelet rich plasma (PRP) concentration device manufacturer confirming that ACTIGEN™ Verified Inductive Bone Matrix maintained greater than 98% cell viability after two hours.
Furthermore, this study further demonstrates that ACTIGEN™ Verified Inductive Bone Matrix is a verified osteoinductive bone graft matrix with appropriate cell- friendly characteristics that is able to deliver and maintain 90% cell viability for a minimum of one (1) hour at room temperature.
- Douglas W. Jackson, Using DBMs in Clinical Orthopedics: Orthopedics Today, October 2005
- Kanim LEA; Houman J; Zhao L; Safai Y; Bae HW; Kropf MA; Delamarter RB: Spine Center, Cedars-Sinai Medical Center, Los Angeles, CA, Composition of Demineralized Bone Matrix-Based Products on Spinal Fusion Rate, Orthopaedic Research Society (ORS) 2012 Annual Meeting
- Dr. Wellington K. Hsu, Interest in Using Stem Cells in Spinal Surgery Increasing: AAOS Now, September 2014
