Pharma Expo The Processing Packaging Event For Pharmaceutical Articles & Analysis: Older
21 articles found
Technological advancements and innovative research have continuously led to the discovery and development of new methodologies in the field of biological and medical sciences. One such breakthrough discovery is bacterial genome editing, which opens a new world of opportunities and challenges in the field of biotechnology and genomics. Understanding Bacterial Genome Editing Bacterial genome ...
Technological advancements and innovative research have continuously led to the discovery and development of new methodologies in the field of biological and medical sciences. One such breakthrough discovery is bacterial genome editing, which opens a new world of opportunities and challenges in the field of biotechnology and genomics. Understanding Bacterial Genome Editing Bacterial genome ...
The pharmaceutical industry has always been at the forefront of innovation, and automation has played a vital role in driving the industry forward. With an increasing demand for pharmaceutical products, there has been a need for faster and more efficient packaging solutions on a commercial scale. Pharmacy packaging automation is an effective solution to meet this demand. Read on to explore ...
The Challenge Originally published in Tablets & Capsules Magazine Capturing and destroying harmful emissions from pharmaceutical processes can be challenging. It’s not because the volatile organic compounds (VOCs) are difficult to destroy using catalytic or thermal techniques. It’s because their concentrations can be so high. These process streams raise safety concerns, both ...
Pharmaceutical formulations consist of active pharmaceutical ingredients (APIs) and excipients. Appropriate excipient selection is critical to the final quality of pharmaceutical products. When designing pharmaceutical preparations, the choice of excipients should not only consider the dosage form factors and excipient functions but also the interaction and compatibility between APIs and ...
Abstract The role of quality systems in pharmaceutical organizations has grown faster than any other function during the last 15 years. During this period, a small group dedicated to traditional Compliance grew and expanded to include Quality Experts in areas such as validation, product release, operations, sterility assurance, and other specialized functions. Creating a deeper quality connection ...
A smart device in the 21st century is an essential tool that bridges the gap between humans no matter where they are in the world. With the constant evolution of technology, we are now able to build a connection and socialise in astonishing ways we would have never imagined decades ago. Though mobile phones are the most common smart devices that people are aware of, modern technology is ...
BySky Labs
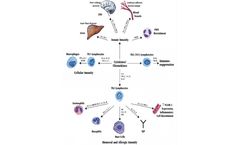
Cytokines are a class of small molecule proteins secreted by cells that mediate and regulate immune processes, and they act in an autocrine, paracrine and endocrine manner. More than 200 types of human cytokines have been identified, which can be generally classified into interleukins, interferons, tumor necrosis factors, colony-stimulating factors, chemokines, growth factors, etc. according to ...
Material: Powder Dust Loadings: Less than 0.5 mg/m3 Installation: LEAK ALERT 65-02 post vacuum pump Filter Type: HEPA (High efficiency particulate air) ...
ByENVEA
The Challenge: Two separate pharmaceutical packaging firms required unique air conditioning technology. In one case, a returning customer requested precisely controlled conditions to provide an exact level of uniform drying for fluid bed dryers used by the manufacturer to coat capsules, pills and caplets. The second pharmaceutical company required a single packaged system containing ...
Clarification for vaccines – the economic and efficient removal of cell debris and negatively charged molecules with FILTRODISC BIO SD and FILTRODISC R2U depth filter modules. The purification of vaccines is a critical step during their complex production process. Cells and cell debris, as well as unwanted contaminants such as host cell proteins (HCPs) and nucleic acids need to be removed, ...
The following is the summary of a noteworthy discussion from the 2019 GMP Academy Master Course – Particle Measuring Systems, Rome, Italy The first GMP Academy Master Course took place from November 26 – 28 at Particle Measuring Systems (PMS) in Italy. International experts met to share their knowledge and expertise of regulatory requirements and practical applications pertaining to ...
Pharmaceuticals and their metabolites are detected on an increasing scale in the aquatic environment. The pharmaceuticals (such as drugs) mostly get into the surface and drinking water through the excretions of humans and animals; however, the wastewater of pharmaceutical production facilities can also be contaminated with active ingredients. The production of pharmaceuticals takes place in two ...
Introduction and Goal Compressed gases are broadly used in medicinal production facilities. A variety of different gases can be introduced into the process at various stages of a product’s manufacture for different reasons. Compressed gases such as air, nitrogen, and carbon dioxide are regularly used in cleanrooms and are frequently employed in purging or overlaying. The cleanliness of ...
Cleaning pharmaceutical processing equipment is challenging. Cleaning methods, soils present, type of manufacturing equipment, surfaces cleaned, choice of cleaning detergent and temperature should all be considered when setting up a cleaning procedure. Cleaning validation methods are required. The entire cleaning process must be standardized and documented according to the FDA’s cGMP ...
On January 13, 2017, slides from the B&C® Consortia Management’s (BC-CM) NMP Producers Group Meeting with the Office of Management and Budget were discussed in the Inside EPA article “Chemical Makers Cite TSCA Listing In Call For EPA To Drop NMP Proposal.” But representatives from Bergeson & Campbell on behalf of NMP producers in a Dec. 5 meeting with EPA and OMB ...
Capturing and destroying harmful emissions from pharmaceutical processes can be challenging. It’s not because the volatile organic compounds (VOCs) are difficult to destroy using catalytic or thermal techniques. It’s because their concentrations can be so high. These process streams raise safety concerns, both when they’re collected in vents and when they reach the final ...
This paper analyses the internationalisation experiences of seven firms in the Indian pharmaceutical industry and identifies the portfolio of capabilities required in the initial phase of internationalisation. It traces the priorities, simultaneity and sequence in the capability development process and identifies the differences between early and late internationalisers. It notes that studies so ...
In this paper we develop a list of critical factors for knowledge management (KM), using different theoretical frameworks. We identified four elements which were common to most models: management promotion, infrastructure, strategy and evaluation. Conducting four case studies from firms in the chemical industry allowed us to compare our factors with empirical data. We found that the involvement ...
This paper analyses the changing R&D process at Japanese pharmaceutical companies, based on a questionnaire survey as well as structured interviews at large Japanese pharmaceutical companies. Japanese pharmaceutical companies have recently engaged in active R&D collaborations with other firms and universities. This paper identifies the factors underlying this trend, finding that all three factors ...