Medical Industry Manufacturers, Suppliers & Companies
-
We are experts in water purification systems and related analysis equipment for 30 years. membraPure was founded over 30 years ago and is still run as a family business. Over the years, membraPure grew and we currently have 31 employees. Our ...
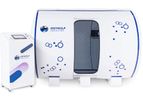
ARACUS - Model IVD - Amino Acid Analyzer
The Amino Acid Analyzer ARACUS IVD is available in two configurations: the ARACUS IVD Classic and the ARACUS IVD Advanced. This choice depends on whether the customer prefers a single-pump or a dual-pump ...
CONTACT SUPPLIER -
We are a team of designers and engineers dedicated to innovate in the development of hyperbaric chambers for personal and professional use. Oxygen is our living, breathing super hero. OXYHELP INDUSTRY is an european hyperbaric device manufacturer ...
Oxylife - Model C - Premium Multiplace Multiplace Hyperbaric Chamber
The OxyHelp OxyLife C Multiplace chamber is ideal for multiple users at the same time. This multiplace hyperbaric oxygen chamber allows either an assistant or an observer inside to monitor the user and assist with the session, electronic functions ...
CONTACT SUPPLIER -
Founded in 1957, the Myron L Company is one of the leading manufacturers of water quality testing instruments in the world. Because of our commitment to customer satisfaction and product improvement, you have our assurance that any changes will be ...
Myron L® - Model Dialysate Meters - Single and Dual Range Conductivity Handheld Dialysate Meter
The choice of hemodialysis professionals for over 40 years, these compact instruments have been designed specifically to test dialysate solutions. By measuring electrical conductivity, they will quickly determine if dialysate concentrations are ...
CONTACT SUPPLIER -
Jacobi Group was founded in 1916 by Ferdinand Adolph Wilhelm Jacobi, today it is made up of three core business units (activated carbons, ion exchange resins and mobile filter units) to serve a wide range of customer needs. With manufacturing ...
-
Since the 1930’s, and now part of Filtration Group, Amafilter® has been designing and manufacturing solid liquid filtration systems for the food & beverages, minerals & minerals industries and renewable fuel industries. Amafilter® specialises in the ...
-
Founded in 1954, the family-owned company Lutz, with over 380 employees in the group, stands for innovative pump and measurement technology as well as system solutions worldwide. In addition to the central company headquarters in Wertheim and ...
Lutz Pumpen | Jesco - Model 6060-000 - Horizontal mount pump - TMR series
The TMR series is a reliable magnetic drive pump that easily handles critical operating conditions due to its patented magnetic axial thrust compensation. * Drive via magnetic coupling with patented magnetic axial thrust compensation * Typical ...
CONTACT SUPPLIER -
PremiumProfessional associationbased in Fargo, NORTH DAKOTA (USA)
IAIA is a forum for advancing innovation, development and communication of best practice in impact assessment. Its international membership promotes development of local and global capacity for the application of environmental assessment in which ...
-
ONDA is the global leader in ultrasound measurement instrumentation and services. Our products are used to acoustically test devices in the medical, industrial, and electronic markets. Over 4,000 hydrophones have been used around the world to ...
Onda - Model Aquas-10 - Fully Integrated Water Conditioning System for Medical Ultrasound Device
The AQUAS-10 is a complete water conditioner designed for use with ultrasound measurements in compliance with Technical Report IEC 62781. Because the quality of acoustic measurement can be significantly affected by various impurities - such as ...
CONTACT SUPPLIER -
For over 30 years, Powder Systems Limited (PSL) has been at the forefront of designing and engineering advanced technology to support process development. We are a globally recognised, award-winning business with expertise in pharmaceutical and ...
PSL - Process Services COPE
C.O.P.E.™ is a hub for innovation and collaboration. Based at PSL’s headquarters in Liverpool, UK, the main focus of the facility is to provide a creative, cutting-edge space for process development, where engineers and manufacturers can ...
CONTACT SUPPLIER -
Welcome to D.R. Sperry & Company, the premier manufacturer of high-quality filter presses for over 150 years. Our filter presses are used by a wide range of industries, including food and beverage, mining, chemical, pharmaceutical, and more. At D.R. ...
-
OPSIS AB is the leading supplier of open-path monitoring systems for air quality monitoring, continuous emissions monitoring (CEM) and process control. OPSIS offers total monitoring solutions and the product line includes both monitoring hardware ...
-
We are a leading European manufacturer of reliable industrial equipment - Made in Germany. Since our foundation in 1912, we have gained over 100 years of experience in reliable engineering solutions that work. Our high reputation and focus on the ...
Koller - Wheelchair Swing
Specially designed for people in wheelchairs. The swing can be used with your own wheelchair (no need to lift it out). Stable and safe construction. Certified safety according to: DIN EN 1176-1 & DIN EN 1176-2. Gentle, even and steady rocking. ...
CONTACT SUPPLIER -
ENVEA manufactures high-precision monitoring systems as well as environmental data processing and reporting solutions with extensive knowledge in the development of complete turnkey solutions. We assist entities in complying with applicable ...
-
Octaform began its life in 1997 as a novel alternative to conventional wall construction. Designed in Canada, the innovation was a stay-in-place concrete wall forming system that reduces energy consumption and extends the life of high-performance ...
Octaform Tempwall - PVC Hygienic Quick Divider Panel
Lightweight and durable, TempWall by Trusscore is an easy to install modular wall system for rapid deployment of temporary rooms and isolation ...
CONTACT SUPPLIER -
Fiberscope.net is a North American-based one-stop resource of inspection cameras for various applications. It's operated by the MEDIT/STRAHL group of companies with its head office located in Winnipeg, Manitoba, Canada, and the US operating branch ...
Model AL-1824 - Arc Lamp - Metal Halide Lamp for MS24/MS48 Light Sources
AL-1824 Arc Lamp assembly is a high-intensity metal-halide bulb that fits MS24 and MS48 metal halide light ...
CONTACT SUPPLIER -
For more than 30 years, Lumex Instruments has been developing and manufacturing laboratory and industrial analytical equipment, instruments, and procedures. We offer both traditional and new ingenious solutions, unique technologies and classical ...
Lumex-Instruments - Model 007QU75 - Microchip RT-PCR Influenza and Covid-19 Detection System
COVID-19 caused by the SARS-COV-2 virus, and influenza by influenza viruses are respiratory tract infections that display overlapping symptoms. Moreover, novel strains of influenza A and B viruses are unpredictable and may cause pandemics like 1918, ...
CONTACT SUPPLIER -
Eliopig is a company created by technicians specialized in the development of biogas and biomethane plants, nitrogen stripping plants and zootechnical equipment for cattle and pig breeding. Since its foundation in 1980, through the constant effort ...
Eliopig - Nitro-Denitro Plants
Eliopig nitro-denitro plant is able to remove up to 70-80% of the total nitrogen, through a process of nitrification and subsequent denitrification. It can be applied both to the digestate and to the zootechnical slurry. What makes our plant ...
CONTACT SUPPLIER -
Galaxy Scientific, an industry pioneer in portable optical Near Infrared spectroscopy, is an agile innovator, enabling organizations to use the power of Near Infrared optical spectroscopy to analyze and authenticate important chemicals and ...
-
Zygo Corporation is a global leader in the design and manufacture of advanced optical metrology systems and ultra-precise optical components and assemblies. Our mission is to enable customer success by delivering innovative precision optical and ...
Zygo - Laser Beam Delivery Systems
Lasers continue to be employed in an ever widening application space. One of the most common challenges is getting the laser from the source to the object, with the right power, size and shape. We develop laser beam delivery systems that employ CW ...
CONTACT SUPPLIER -
Maschinenfabrik Gustav EIRICH was founded in 1863. Originally a mill workshop, it has developed over the years into a group of companies with worldwide operations. Until today the EIRICH Group remains committed to the governing principle once ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you