Edwards Lifesciences Corporation products
Devices - Transcatheter Heart Valves
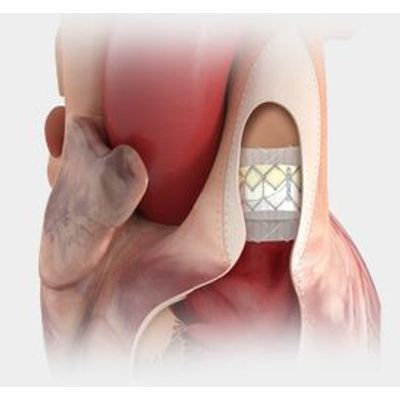
Edwards Sapien - Transcatheter Heart Valves for Predictable Deployment
The first transcatheter heart valve approved for pre-stented transannular patches. Also approved for use in the pulmonic position in conduits and valve-in-valve
Devices - Transcatheter Heart Valves - transcatheter Aortic
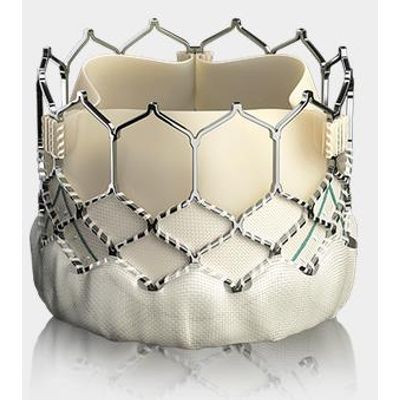
Sapien - Ultra Transcatheter Heart Valve
Built on the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patient needs in mind.
Sapien - Transcatheter Heart Valves
Builds upon the proven strengths of the SAPIEN valve with 5 years of durability. The SAPIEN 3 valve is the only valve approved for valve-in-valve procedures in both the aortic and mitral positions, allowing patients at high or greater surgical risk to avoid an additional open heart procedure.
Sapien - Transcatheter Heart Valves for Pre-stented Transannular Patches
Designed for predictable deployment. The SAPIEN XT valve may be used in the aortic position via a broad range of access routes - transfemoral, transapical and transaortic.
Devices - transcatheter Valve Repair
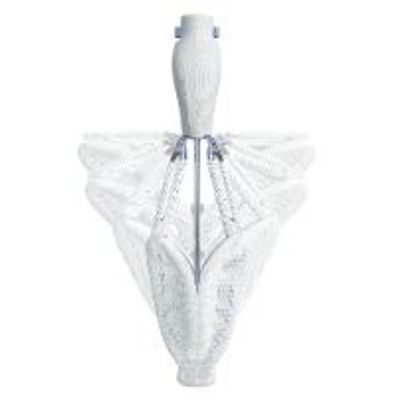
Edwards - Pascal Transcatheter Valve Repair System
In the early days of transcatheter mitral valve repair, the goal was a successful procedure that reduced mitral regurgitation (MR) to less than 3+; residual MR grade 2+ was considered acceptable.1,2 Compelling new data on more than 5400 patients show that residual MR grades 0–1+ are significantly associated with superior patient outcomes when compared with residual MR 2+, including reduced mortality and rehospitalisation.3–8