- Home
- Companies
- LIQID Medical
- Products
LIQID Medical products
LIQID - Glaucoma Device
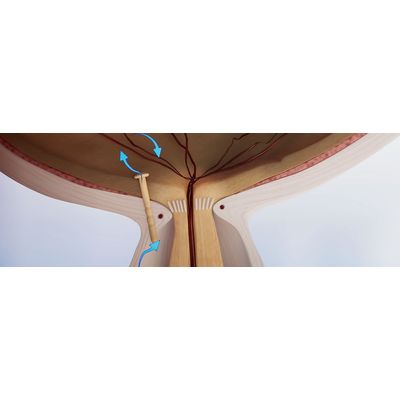
The iFlow uses the traditional subconjunctival fluid drainage pathway. The device however incorporates innovative design features to provide surgeons with a customisable, individualised treatment solution to prevent complications and improve clinical outcomes. The device is designed to expand the LIQID Medical portfolio to leverage the MIGS glaucoma devices market for patients with earlier stage glaucoma. The device is earmarked for commercialisation readiness in 2023.
LIQID - Vitreoretinal Glaucoma Device
The iPortVR is a vitreoretinal glaucoma device uses the same novel technology as the OptiShunt, but has been designed for use in patients undergoing vitreoretinal (VR) surgery. The iPortVR is the first ever glaucoma device designed for use during vitreoretinal(VR) surgery, which is commonly associated with glaucoma. Our patented design makes the iPortVR compatible for use with all VR surgical techniques.
OptiShunt
LIQID - Flagship Device
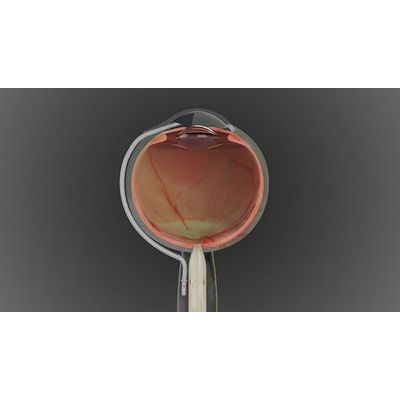
The OptiShuntV1 is a proof of concept device designed to evaluate the safety, feasibility, and efficacy of patented technology for the treatment of glaucoma. The device is manufactured using world-class silicone micro moulding techniques according to ISO13485 guidelines and includes a custom shunt insertion system. Clinical trials are currently underway in the GOSSIP study being conducted at Groote Schuur tertiary academic Hospital, the home of the world’s first heart transplant.
LIQID - Flagship Device
The OptiShuntV2 is the follow-on device which incorporates design optimisation changes and is set for clinical trials kicking off in January 2022. The device includes a two-part construction and an upgraded shunt insertion system to make the device simpler, safer, and even more effective.