MV BioTherapeutics SA products
Technology
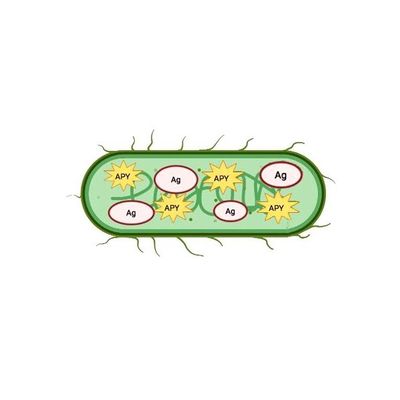
ApyraVax - Live Attenuated Typhoid Vaccine
The ApyraVax technology exploits the live attenuated typhoid vaccine as vector to deliver orally an enzyme that breaks down ATP (apyrase) together with desired antigen/s. The innovative feature is the unique ability to generate high-affinity secretory IgA that are protective towards the target infectious agent.
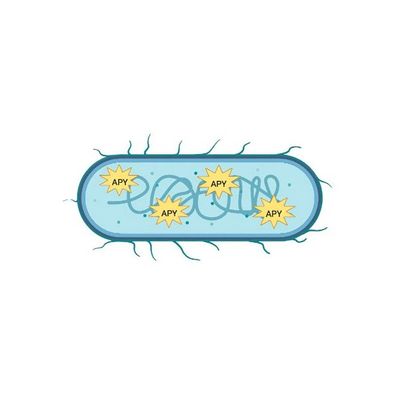
ApyraMed - Biotherapeutic Technology
The ApyraMed technology exploits a recombinant live biotherapeutic that promotes adaptive intestinal homeostasis. In cancer patients, it enhances immune system competence, whereas in dysbiosis safeguards the intestinal ecosystem.
Technology - Programs
ApyraVax - Clostridioides Difficile Infection (CDI)
Clostridioides difficile infection is a world-wide serious and deadly disease. Clostridioides difficile infection (CDI) is a nosocomial, antibiotics associated infectious diarrhea characterized by a high mortality rate, in particular in people above the age of 65. Current treatments include antibiotics and fecal transplantation. Recurrence rates are 20-30% and deaths in the US over 29’000 every year. The total cost per patient is extremely high. ApyraVax is suitable for continuous dosing, exploits the already marketed and safe Salmonella Ty21a vaccine strain (Vivotif®) and induces high-affinity secretory IgAs that ensure protection from CDI.
ApyraMed - Antibiotic-induced Dysbiosis
Antibiotic-induced dysbiosis exposes the organism to various medical conditions. The intestinal mucosal surface is inhabited by a complex commensal microbiota that ensures intestinal homeostasis and organism’s physiologic functions. Antibiotic treatments can severely affect microbiota diversity and cause dysbiosis, thereby exposing the host to pathophysiological consequences, including intestinal inflammation, susceptibility to enteric infections, metabolic alterations and a number of systemic effects. ApyraMed promotes microbiome recovery and selection of beneficial bacteria after antibiotic treatment. This results in powerful control of antibiotic-induced enteric infections and metabolic alterations.
ApyraMed - Cancer Immunotherapy with Immune Checkpoint Inhibitors (ICIs)
Cancer immunotherapy has therapeutic efficacy in a limited number of patients. Cancer immunotherapy with immune checkpoint inhibitors (ICIs) increases anti-tumor immunity by blocking molecules that suppress anti-tumor activity. However, only a limited fraction of patients benefits from the therapeutic response obtained with these biologic drugs. Therefore, factors that could improve the therapeutic outcome are needed. In patients responsive to the therapy with ICIs, the tumor microenvironment is replenished with fresh T cells with self-renewing capacity from sites outside the tumor. This phenomenon appears to be a key factor in explaining the clinical benefit of ICIs. ApyraMed has shown to promote the effective generation of self-renewing anti-tumor cytotoxic T cells and synergize with ICIs in controlling tumor growth, thus resulting in superior survival in experimental models of solid tumors.