- Home
- Companies
- Origin, Inc
- Products
Origin, Inc products
Clinical Pipeline
Ionoderma - Dermatological Clinical Pipeline
Dermatological applications for Nitric Oxide. Our initial target indication is onychomycosis . Nitric Oxide Treatments for Dermatological Applications.
IonoStim - Wound Healing Clinical Pipeline
IonoStim is our most advanced Program, the focus of which is wound healing. We are leveraging NO’s potent antimicrobial activity along with its ability to stimulate the body’s natural healing mechanisms. Skin is the largest organ in the human body, and so it is not surprising that costs associated with treating wounds account for a large proportion of global healthcare spend.
Ionodent - Dental Applications Clinical Pipeline
Designed for dental applications such as periodontitis and implantitis. The focus here is on NO’s antimicrobial properties, with specific design features that enable intra-oral application.
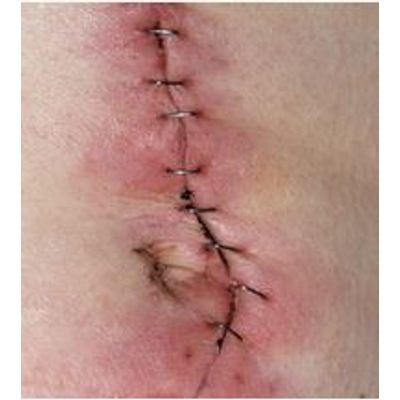
Ionosite - Surgical Site Infection Clinical Pipeline
SSIs represent a significant burden in terms of patient morbidity and mortality, and add significant costs to health systems and service payers worldwide (49). CDC has reported costs of $5 billion, but estimates elsewhere in the literature have reached as high as $29 billion per year (48).
Technology
Origin - Nitric Oxide Technology
Nitric Oxide (NO) was first prepared in 1620 and first studied in 1720. It was poorly understood until the late 1900s. The discovery of the role of NO as fundamental to biologic function was so remarkable that hundreds of research papers led Nitric Oxide to be proclaimed "molecule of the year" in 1992 by the journal Science. Three NO researchers shared the 1998 Nobel Prize in Physiology or Medicine for their discovery that what had previously been known as EDRF (endothelium-derived relaxing factor) was, in fact, NO (13,14).