- Home
- Companies
- Selenium Medical
- Products
Selenium Medical products
Selenium Packaging
The Revolutionary Sterile Double-Blister
SoBliss® allows new blister packaging vision. Implants can now be packaged in 360° transparent & rigid “double-blister”, that provides a better match in terms of identification, handling, and storage. It also secures transfer procedure (no aseptic faults during surgeries thanks to its “No Touch” approach capability. SoBliss® is suited for a wide range of implants in a longitudinal and flat shape or slightly curved implants. All raw materials have been analyzed and chosen for their ability to be used in the medical field to package or be in contact with medical device and are compliant with ISO 10993-1/5/11. SoBliss® Sterility can be maintained over a five years shelf life. Thanks to the hinge and vertical all-around sealing, a puncture roof Tyvek® system has been created.
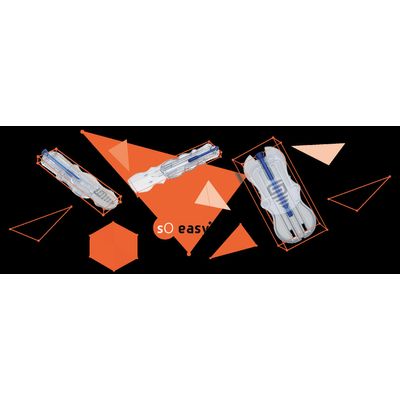
Smart Holder 100% No Touch
SoEasy® has been developed in order to improve screw protection in a cost-efficient packaging. The holder’s shape prevents pin holes in the sterile barrier system and offers NoTouch features. SoEasy® allows an intuitive use, suitable for right & left handed persons. SoEasy® is able to hold several types of implants (dental, trauma, extremities…). 2 sizes (M & L+) are available to cover a Ø range from Ø1.6 to Ø8.5mm and for a length between 10mm and 110mm.
Selenium Surface Treatment
Protective Surface Treatment
3 possibilities for colored, resistant and protected titanium. We offer titanium anodisation through processes developed and validated by our expert team as per IQ OQ PQ PPQ, in accordance with the requirements of ISO 13485 and 21 CFR820s. This electrochemical processes leading to formation of a titanium oxide layer on the surface of products, granting the materials enhanced resistance to wear and corrosion. In addition to protecting medical devices, these processes make it possible to color them (without coloring agents or pollutants) thanks to the phenomenon of interference and crystallographic orientation of the oxide formed, while enhancing their biocompatibility through modification of surface polarity. We place our in-house expertise at your service via our three titanium anodisation solutions ensuring reproducible results coupled with the perfect biocomptability of the implants.
Promotive Surface Treatment
StarSurf® is a surface treatment process enabling to create a rough structure on the surface of implants made of titanium and titanium alloys thus improving their biological performance of osseointegration. Implants surface finishing plays a crucial role in the success of an implantation surgery. As a matter of fact, the first contact between the implant and the patient’s body is a key step towards its success. StarSurf® etching process was originally developed for dental application on titanium and titanium alloy Ti6Al4V ELI implants aiming at improving their biocompatibility and therefore patient comfort and safety. StarSurf® etching process also applies to other fields such as orthopaedics, maxillofacial implants or other customer demands.
Traceability Surface Treatment
We offer high-precision laser marking validated as per IQ OQ PQ PPQ principles, in accordance with the requirements of ISO 13485 and 21 CFR820s. Our team is capable of marking all types of metallic (titanium, stainless steel, etc.) and polymeric (PEEK, etc.) materials. Our expertise in this process makes it possible to produce marking of different sizes, typefaces, curvatures, orientations and colors. Our lasers combine sensitivity and precision while ensuring the full integrity of marking so guaranteeing the traceability of your medical devices.