T-Cure BioScience, Inc. products
Pipeline
T-Cure - HLA-A2 Haplotype
Using its iSORT platform, T-Cure identified 806TCR, an NY-ESO-1-specific TCR restricted to HLA-A2 haplotype. The 806TCR is in IND-enabling studies. NY-ESO-1 has been demonstrated clinically as a suitable target for TCR therapies for multiple solid tumors. T-Cure is currently exploring partnerships in developing “off-the-shelf” approaches to utilize this all natural but high affinity TCR in the clinic. T-Cure believes the 806TCR holds great promise as a component of such therapies.
T-Cure - Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1)
The Kita-Kyushu Lung Cancer antigen-1 (KK-LC-1) is a cancer/testis antigen (CTA) that is expressed in multiple solid tumors while restricted to germ line cells in normal tissues. T-Cure estimates that KK-LC-1 is expressed in 80% gastric cancer, 75% triple negative breast cancer (TNBC), 45% non-small cell lung cancer (NSCLC) and 40% cervical cancer. 820TCR was isolated from a complete responder patient after Tumor Infiltrating Lymphocytes (TIL) therapy. 820TCR based therapy is undergoing a Phase 1 clinical study at the National Cancer Institute (NCT05035407). T-Cure has licensed the KK-LC-1 TCR from the NIH and has an CRADA collaboration with the NIH to develop KK-LC-1 TCRs in solid tumors.
T-Cure - Human Endogenous Retro-Virus (HERV)
The HERV-E target is one of the quiescent Human Endogenous Retro-Virus (HERV) sequences that are turned on during tumor development. Its expression in renal cell carcinoma (RCC) is highly selective, with no transcripts detected in any normal tissues. It is expressed in 85% of clear cell RCC (ccRCC). The TCR molecule was identified in a responding patient, following allogeneic stem cell therapy. Currently there is an ongoing investigator-initiated trial at the NIH for metastatic ccRCC patients with progressive disease following anti-angiogenic drug and nivolumab (anti-PD1) therapy (NCT03354390). T-Cure has licensed the HERV-E targeted TCR from the NIH and has an active CRADA collaboration with the NIH to develop HERV-E TCRs in solid tumors.
Technology
T-Cure - Isolation of Optimal Reverse Immunology Generated TCR (iSORT) Platform Technology
Our exclusive iSORT (Isolation of Optimal Reverse Immunology Generated TCR) platform is able to isolate TCRs against unique selected tumor-associated intracellular and extracellular antigens and neoantigens in the context of an HLA molecule, allowing us to focus on specific cancers and patient populations based on their genetic makeups. Using this technology, we are able to isolate TCR candidates capable of targeting antigens presented by both class I and class II HLA molecules.
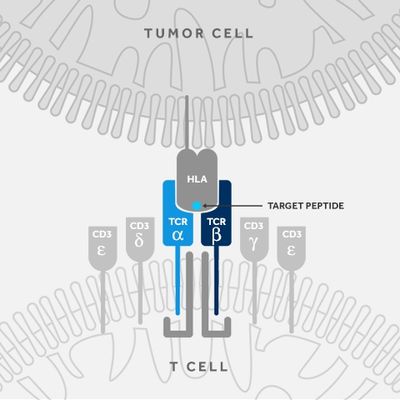
T-Cure - Receptor Therapy T Cell
T cell engineering with TCRs is a growing area of immuno-oncology with recent breakthroughs in clinical settings. The T-Cure approach involves identifying and isolating a high avidity TCR which specifically recognizes an intracellular or cell-surface target in solid tumors. This TCR is then typically introduced into a viral vector that can deliver the TCR into the patients’ own T cells in an ex vivo setting. After successful genetic modification of the T cells in this way, T cells are re-introduced into the patient, and render effective killing of tumor cells in vivo.