

- Home
- Companies
- Ariana Pharma
- News
- Anavex Life Sciences and Ariana Pharma ...
Anavex Life Sciences and Ariana Pharma present positive phase II clinical and biomarker data at the AD/PD 2022 International Conference
Ariana Pharma, the eXplainable Artificial Intelligence clinical development Company, announced today the presentation of Phase 2 clinical biomarker data from the ANAVEX2-73-PDD-001 Parkinson’s Disease Dementia (PDD) study at the AD/PD 2022 International Conference.
MDS-UPDRS Total score improved significantly for patients treated with ANAVEX®2-73 high oral once-daily dose compared to placebo. The improvement is clinically relevant, corresponding to a relative improvement of 18.9% over 14 weeks. Balanced and global improvements were observed within all MDS-UPDRS sub-scores Part I-IV.
SIGMAR1 mRNA expression biomarker, previously identified using Ariana’s eXplainable Artificial Intelligence (xAI) KEM® technology, was significantly increased in ANAVEX®2-73 treated patients vs. placebo over the course of treatment (p=0.035). In addition, this biomarker was significantly associated with improvements of MDS-UPDRS scores and cognitive efficacy endpoints CDR system.
Age, certain neurophysiological measures, and high systolic blood pressure at baseline were identified with an increased response for the endpoints.
This finding builds on the KEM® AI-driven precision medicine clinical development platform of ANAVEX®2-73, where the SIGMAR1 mRNA expression biomarker as previously identified as a potential biomarker (Hampel et al, Alzheimer Dement (N Y). 2020; 6(1): e12013) is characterized across multiple indications, including Alzheimer’s Disease and Rett syndrome.
“The reported strong positive biomarker and clinical results in Parkinson’s Disease Dementia, building on previously reported positive results in RETT syndrome and Alzheimer’s disease, further illustrate the power of KEM® Explainable AI to accelerate precision medicine drug development building on in-depth understanding of the intersection between SIGMAR1 molecular pathways and disease biology” said Mohammad Afshar, M.D., Ph.D., Chief Executive Officer of Ariana Pharma.
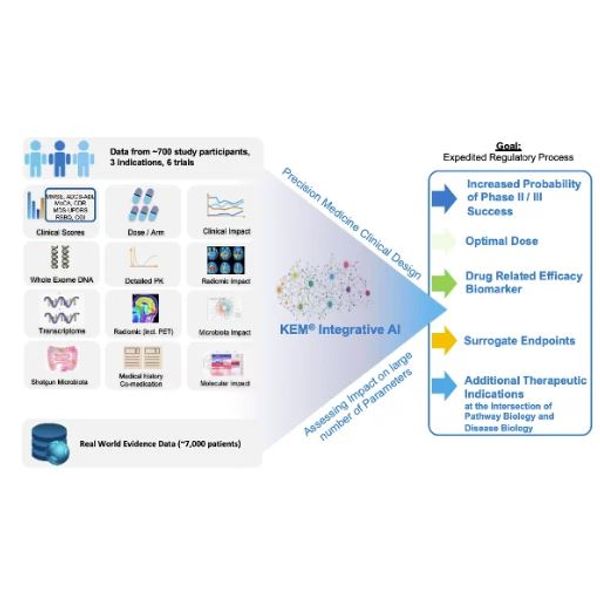
Ariana Pharma is a leading Artificial Intelligence (AI) drug development company. Using its KEM® Artificial Intelligence (xAI) technology, Ariana helps its partners introduce personalized medicine clinical trial design into their protocols and optimize clinical endpoints, identify biomarkers of therapeutic response and potential synergistic therapies.
Ariana routinely collects and combines clinical data with omic data, immunological readouts (such as Fluorescence-Activated Cell Sorting (FACS)), microbiota, Patient Reported Outcomes (PRO) as well as Real World Evidence (RWE) data. Combining advanced data analytics, drug development, and regulatory expertise, Ariana helps translate findings into innovative clinical development plans and regulatory approvals.
With a growing number of successful therapeutic development programs, KEM® is an FDA-assessed technology that systematically explores combinations of biomarkers, producing more effective biomarker signatures for precision medicine. Ariana has developed Onco KEM®, the most advanced, clinically tested, oncology therapeutic decision support system. Founded in 2003 as a spin-off of the Institut Pasteur, Paris, France, the company operates a subsidiary in the United States since 2012.
