

- Home
- Companies
- CardioWise Inc.
- News
- CardioWise™ Receives ISO 13485:2016 ...
CardioWise™ Receives ISO 13485:2016 Clearance for Design, Development, and Marketing of Software as a Medical Device (SaMD) For the Medical Device Industry
CardioWise, Inc., is pleased to announce that Perry Johnson Registrars, Incorporated has audited the CardioWise Quality Management System (QMS) and determined CardioWise is in conformance with ISO 13485:2016. Perry Johnson Registrars Certificate C2022-02910 was issued July 16, 2022, and represents the first step in the process of obtaining a CE mark and Medical Device Registration for the European Union (EU) that will allow CardioWise to market its products there.
This follows the company’s FDA 510(k) Clearance for CardioWise SQuEEZ that allows the company to commercialize its software in the US. SQuEEZ is based on the ability in high resolution cardiac CT scans to track points on the endocardium as they move during the contraction of the heart to allow quantitative assessment of regional myocardial heart wall motion. The analysis of four dimensional SQuEEZ parameters has the potential to improve the ability to quantify accurately the regional myocardial contractile function (ability of the heart to pump blood) through the heart muscle of the left ventricle.
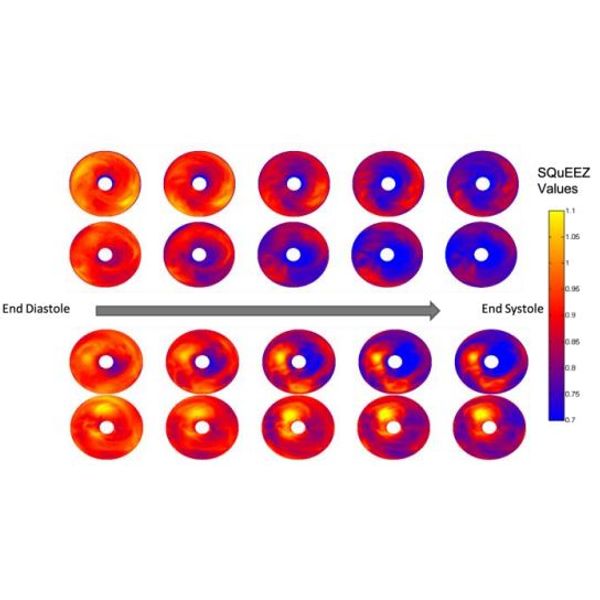
The image at the left displays the left ventricular SQuEEZ “bullseye” maps of two subjects with normal hearts (top) and two patients with infarcted hearts indicating cardiovascular disease. The analysis clearly shows large abnormal yellow areas indicating regions of the endocardial wall that are not moving normally due to the damage. Cardiac CT combined with SQuEEZ analysis software can provide clinicians with a normalized measurement to determine the answer to the first and most important clinical question that a cardiac surgeon needs to know for a patient who presents with Cardiovascular Disease (CVD) symptoms—“Is the contractile function normal or not normal?” SQuEEZ allows the surgeon to make a more informed decision about the proper intervention for the patient, whether it is a drug, surgery or medical device, and then follow the patient’s recovery and progress after the procedure, non-invasively and with extremely low exposure to radiation. The software is an intuitive, quantitative method to classify LV contractile function that has the potential to provide significant improvement in clinical decision-making relative to the inconsistent, qualitative tests used today. The software provides the ability to both quantify and localize left ventricular (LV) contractile dysfunction. This will improve clinical outcomes by addressing limitations of the current nonquantitative metrics of regional LV function. The software creates an objective standard that increases accuracy by reducing the user variability that has plagued interpretation of LV functional analysis. The simplicity of interpreting and displaying these results will increase patients’ understanding of their coronary artery disease.
Jack Coats, CEO of CardioWise, Inc. said, “We are very pleased that the ISO 13485:2016 Certification has been achieved for the company’s QMS. I congratulate the CardioWise Engineering and Regulatory team, led by Geoffrey Dalbow, Chief Technology Officer of CardioWise, for its efforts over the past months to develop and document its Standard Operating Procedures within the QMS to achieve this extraordinary task. This achievement opens the door to the next steps that will allow the company to market SQuEEZ in the EU. SQuEEZ analysis will eventually provide caregivers in the EU with an easy-to-read, quantitative 4D color model that allows physicians to make better, more informed decisions about their patient’s care.”
CardioWise is commercializing patented, non-invasive Cardiac CT analysis software that produces a quantified 4D image model of the human heart, called Stretch Quantifier for Endocardial Engraved Zones (SQuEEZ). CardioWise heart analysis software combined with cardiac CT is a single diagnostic test that is able to provide quantitative analysis of the myocardium, arteries and valves with an unprecedented level of detail. It has the opportunity to become the new gold standard of care for heart health analysis. CardioWise is a VIC Technology Venture Development portfolio company.
