

- Home
- Companies
- Hemostemix Inc.
- News
- Hemostemix’s Boots on the Ground in ...
Hemostemix’s Boots on the Ground in Florida
- Direct engagement with podiatrists, cardiologists, and vascular specialists.
- On-site meetings with former Phase II trial sites to transition trial experience into clinical adoption.
- Educational outreach to clinic owners and healthcare providers on patient eligibility, treatment protocols, and data collection (outcomes of treatments).
- Ongoing follow-ups with regulators, including the FDA, with respect to the data generated from treatment outcomes.
- Peripheral Arterial Disease (PAD)
- Chronic Limb-Threatening Ischemia (CLTI)
- Angina
- Ischemic Cardiomyopathy
- Non-Ischemic Dilated Cardiomyopathy
- Congestive Heart Failure
- Total Body Ischemia
- Podiatrists & Vascular Surgeons
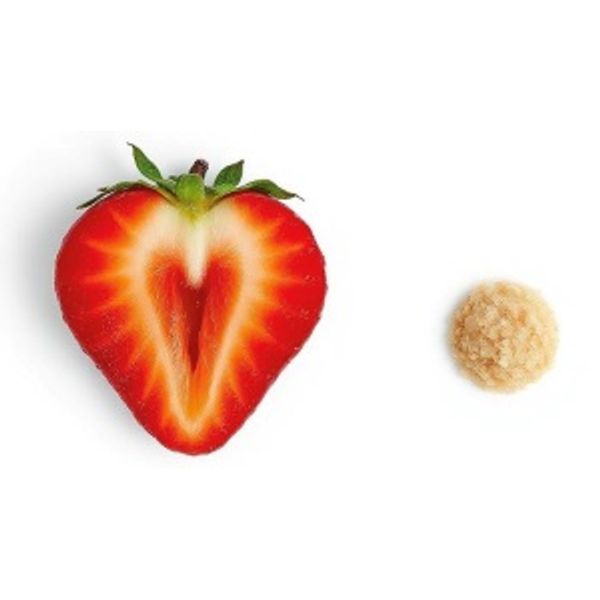
Ulcer size in the treated group decreased from a mean of 1.46 cm2 (the size of a wild strawberry) to 0.48 mm2 (p = 0.01) (the size of a grain of sand) by 3 months. There was no significant decrease in the size of the ulcers of the placebo group (p < 0.54).
- In three published trials, including Phase II, VesCell™ generated new blood vessels, restored circulation, healed ulcers, and reduced rates of amputation.
- Cardiologists and Thoracic Surgeons
- In three published studies of 41, 106, and 53 subjects, respectively, VesCell™was safe, and clinically relevant. It improved overall ejection fraction significantly, improved exercise capacity and quality of life.
- In the treatment of chronic stable angina (24 subjects), VesCell™reduced severe angina symptoms, improved exercise capacity, improved six minutes walk test results, and generated clinical improvement in all patients.
Hemostemix is an autologous stem cell therapy platform company, founded in 2003. A winner of the World Economic Forum Technology Pioneer Award, the Company has developed, patented, is scaling and selling autologous (patient’s own) blood-based stem cell therapy, VesCell™ (ACP-01). A recent peer-reviewed article in Cells (June 29, 2025) provides the scientific foundation for how ACP-01 and NCP-01 may enhance brain-computer interface performance by reducing inflammation, fostering angiogenesis and synaptic plasticity, and potentially extending implant longevity. Hemostemix has completed seven clinical studies of 318 subjects and published its results in 11 peer reviewed publications. ACP-01 is safe, clinically relevant and statistically significant as a treatment for peripheral arterial disease, chronic limb threatening ischemia, non ischemic dilated cardiomyopathy, ischemic cardiomyopathy, congestive heart failure, and angina. Hemostemix completed its Phase II clinical trial for chronic limb threatening ischemia and published its results in the Journal of Biomedical Research & Environmental Science. As compared to a five year mortality rate of 50% in the CLTI patient population, UBC and U of T reported to the 41st meeting of vascular surgeons: 0% mortality, cessation of pain, wound healing in 83% of patients followed for up to 4.5 years, as a midpoint result.
