Refine by
Diabetic Prediabetic Patients Articles & Analysis: Older
116 news found
This year Protheragen-ING has extended its portfolio of Active Pharmaceutical Ingredients (APIs) to include several hundred new varieties, catering to a broad spectrum of therapeutic needs such as anti-tumor, hypoglycemic, antipsychotic, bronchiectasis, and antihypertensive treatments. Such growth represents Protheragen-ING’s commitment to support researchers worldwide in their quest of ...
We are delighted to welcome Roczen to the community of healthcare organisations leveraging Vantage Software! We look forward to working together and helping them deliver exceptional care and support to those living with chronic conditions. Book a Free Demo ...
New Subgroup and further analyses from PULSAR, PHOTON and CANDELA provide insights into durability results of extended treatment intervals, patient characteristics as well as efficacy and safety of intravitreal aflibercept 8 mg In total, 18 presentations on aflibercept 8 mg and Eylea (aflibercept 2 mg) reinforce Bayer’s commitment to advancing treatment of patients with exudative retinal ...
ByBayer AG
European Commission granted approval for a label update to extend the indication of Kerendia™ to early stages of chronic kidney disease (CKD) associated with type 2 diabetes (T2D) and to include findings from the Phase III FIGARO-DKD cardiovascular (CV) outcomes study FIGARO-DKD included approximately 7,400 patients across a broad range of disease severity, including stages 1-4 CKD ...
ByBayer AG
Nautilus Biotechnology, Inc. (NASDAQ: NAUT; or “Nautilus”), a company pioneering a single-molecule protein analysis platform, and the Translational Genomics Research Institute (TGen), part of City of Hope, today announced a partnership to explore the utility of the Nautilus platform by studying specific protein targets in diffuse intrinsic pontine glioma (DIPG), a rare and often fatal ...
CHMP opinion is based on the results from the Phase III FIGARO-DKD cardiovascular (CV) outcomes study in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D), which included approximately 7,400 patients across a broad range of disease severity, including stages 1-4 CKD associated with T2D The positive data from FIGARO-DKD demonstrated that finerenone significantly reduced the ...
ByBayer AG
Inversago Pharma Inc. (“Inversago”), a clinical stage biotech company with a unique portfolio of peripherally-acting CB1 inverse agonists, today announced that the first patient has been dosed with INV-202 in a Phase 2 clinical trial in subjects with diabetic kidney disease (DKD). The Phase 2 trial of INV-202, a first-in-class, peripherally-acting CB1 inverse agonist, is a ...
An estimated 415 million people are living with diabetes in the world. Diabetes is a chronic disease, that causes blood sugar levels in your body to become too high, either due to your body not being able to respond properly to insulin or due to your body not being unable to produce any at all. If left untreated, this can lead to serious complications such as heart attack, stroke, obesity, eye ...
Apollo Medical Holdings, Inc. ("ApolloMed," and together with its subsidiaries and affiliated entities, the "Company") (NASDAQ: AMEH), a leading physician-centric, technology-powered healthcare company focused on enabling providers in the successful delivery of value-based care, today announced that ApolloMed received certification from the National Committee for Quality Assurance ("NCQA") for ...
Destiny Pharma plc (AIM: DEST), a clinical stage innovative biotechnology company focused on the development of novel medicines that can prevent life-threatening infections, is pleased to announce the commencement of an Investigational New Drug (IND) enabling safety study with its novel XF-73 Dermal formulation. This study is the second of two planned preclinical safety studies of the XF-73 ...
Key Research Survey Findings: 52 percent of healthcare providers agreed motivation is a major barrier to patients adhering to blood glucose monitoring compared to only 10 percent of people with diabetes who agreed motivation is a major barrier. Healthcare providers claimed they are recommending blood glucose management apps to only 25 percent of their patients, while 55% of patients who ...
ByLifeScan
Aflibercept 8 mg demonstrated non-inferior vision gains to Eylea® (aflibercept 2 mg) with 83% of patients with neovascular (wet) age-related macular degeneration (nAMD) and 93% of patients with diabetic macular edema (DME) maintaining dosing intervals of 12 weeks or longer through week 48 Superior fluid control in nAMD and robust disease control through to week 48 in nAMD and DME In both ...
ByBayer AG
PharmAbcine Announces Poster Presentations on Its anti-VISTA Antibody Candidate at SITC 2022 DAEJEON, South Korea, October 17, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of antibody therapeutics, announced today that the Company will present preclinical data of PMC-309, one of the Company’s first immuno-oncology ...
Microbion Corporation today announced that the company presented a poster highlighting the effect of pravibismane on bacterial energetics in Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Mycobacterium avium species at ASM Microbe 2022 in Washington, DC. The objectives of these studies were to further understand the mechanism of action of pravibismane, in both planktonic and ...
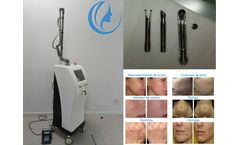
CO2 fractional laser resurfacing machine treatment can quickly improve skin texture, firm skin, improve large pores, and make skin smooth and tender like water. CO2 fractional laser technology is now the world's leading technology for wrinkle removal and skin rejuvenation. Through pulsed fractional laser, laser beauty has been raised to a new level. Bvlaser is a professional CO2 fractional laser ...
The last patient in the investigator-initiated clinical trial DIAGNODE-B has received its additional injection (“booster”) of the therapeutic diabetes vaccine Diamyd®. The trial includes 6 patients with Type 1 diabetes who earlier participated in the DIAGNODE-1 or DIAGNODE-2 trials and who carry the genetic HLA DR3-DQ2 haplotype. DIAGNODE-B (B for “booster”) assesses ...
Updated 12-month results from the open-label investigator-initated Phase II clinical trial GADinLADA that assessed three intralymphatic injections of the therapeutic diabetes vaccine Diamyd® in individuals diagnosed with Latent Autoimmune Diabetes in Adults (LADA) were presented today at the European Association for the Study of Diabetes (EASD) conference in Stockholm, Sweden. The updated ...
New post-hoc analyses from the prespecified pooled analysis FIDELITY will provide insights into the effects of finerenone on kidney and cardiovascular outcomes by use of glucagon-like peptide-1 receptor agonists (GLP-1RA) at baseline, as well as by baseline HbA1c, HbA1c variability, and diabetes duration in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) An exploratory ...
ByBayer AG
U.S. Food and Drug Administration (FDA) granted label update for Kerendia (finerenone) to include findings from the Phase III FIGARO-DKD cardiovascular (CV) outcomes study in patients with chronic kidney disease (CKD) associated with type 2 diabetes (T2D) FIGARO-DKD investigated the efficacy and safety of finerenone versus placebo in addition to standard of care on the reduction of CV ...
ByBayer AG
In the September 2022 issue of JACC journals (https://www.jacc.org/doi/10.1016/j.jacc.2022.06.044), the FAVOR III China Study Group published a sub-study that was aimed to ascertain whether the beneficial outcomes of QFR® guidance for lesion selection during PCI is affected by diabetes status. From the total of 3825 patients enrolled, 1295 (33.9%) had diabetes. The study confirmed that the ...