

- Home
- Companies
- Synairgen plc
- News
- Topline results from Phase 3 SPRINTER ...
Topline results from Phase 3 SPRINTER trial
Synairgen plc (LSE: SNG), the respiratory company developing SNG001, a formulation for inhalation containing the broad-spectrum antiviral protein interferon beta, today announces that the international Phase 3 SPRINTER trial of SNG001 in patients hospitalised with COVID-19 did not meet its primary or key secondary efficacy endpoints. SNG001 demonstrated a favourable safety profile and was well tolerated in this population.
Richard Marsden, CEO of Synairgen, commented: "While we are disappointed by the overall outcome, SNG001 has been administered to hospitalised patients on top of standard of care which changed substantially between our Phase 2 and Phase 3 trials. This improvement in patient care may have compromised the potential of SNG001 to show a clinical benefit in respect of the endpoints for this study, which were not met. Despite this we have observed an encouraging trend in prevention of progression to severe disease and death, which we strongly believe merits further investigation in a platform trial. We are now analysing the full dataset to better understand all the findings."
"In the meantime, we eagerly await the Phase 2 data from the US NIH ACTIV-2 trial in home- based COVID-19 patients, and that trial`s larger, follow-on Phase 3 study, as part of the development path for SNG001."
Efficacy
A total of 623 patients were randomised to receive SNG001 (n=309) or placebo (n=314) in addition to standard of care (SOC). The primary analysis was conducted in the intention-to-treat population (ITT; all randomised patients). Data for the per protocol population (PP) is also shown. The PP population excludes patients with major protocol violations that may have confounded the results.
Primary Endpoints
Regarding the primary endpoints (Table 1), patients who received SNG001 were no more likely to be discharged from hospital than patients who received placebo, and patients who received SNG001 were also no more likely to recover to `no limitation of activities` than patients who received placebo, in both the ITT and PP populations. The evolution in standard of care over the course of the pandemic (for example, 87% of patients in this trial received systemic corticosteroids for COVID-19 at baseline whereas none did in the Phase 2 study of SNG001 in COVID-19[1]) may have compromised the potential of SNG001 to show a clinical benefit in respect of the primary endpoints for this study.
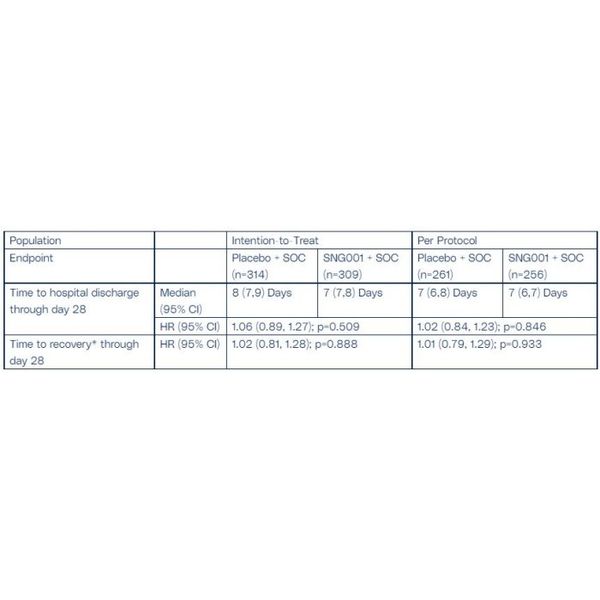
* to `no limitation of activities` on the WHO ordinal scale for clinical improvement (OSCI). HR =Hazard ratio; 95% CI = 95% confidence intervals. The per protocol population excludes patients with major protocol violations that may have confounded the results. These violations included receiving fewer than 2 full doses in the first 3 days, ongoing SARS-CoV-2 infection for more than 3 weeks prior to randomisation, not having a positive SARS-CoV-2 test result at screening, patients kept in hospital for reason other than the severity of their COVID-19 and patients who were not escalated to advanced respiratory support despite clinical need.
For the key secondary endpoints (Table 2), there was a trend in favour of SNG001 for the endpoint measuring progression to severe disease or death within 35 days of randomisation with a 27% and 36% relative risk reduction, for the ITT and PP populations respectively, in the proportion of patients who were treated with SNG001 compared to patients on placebo.
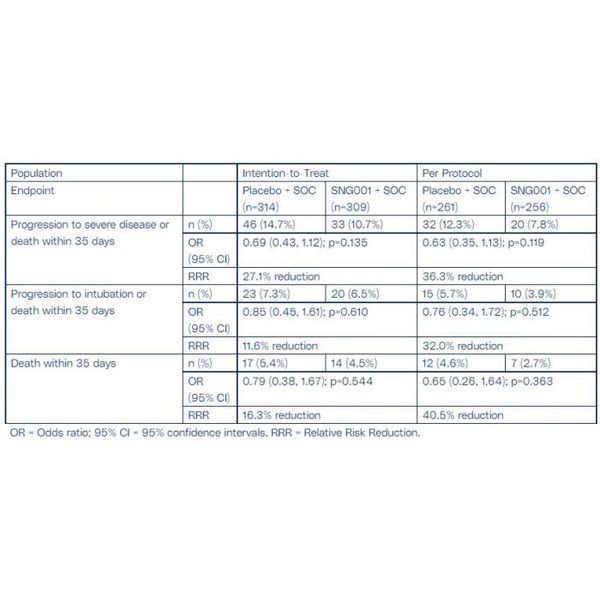
Safety
SNG001 was well tolerated in the SPRINTER trial with a favourable safety profile consistent with previous studies. The proportion of patients with any serious treatment-emergent adverse events was 12.3% on SNG001 and 18.2% on placebo. The proportion of patients with any treatment-emergent adverse events related to study treatment was 22.3% on SNG001 and 25.7% on placebo.
Next steps
Synairgen will now review the study`s full dataset to better understand the detailed results and implications for development for SNG001 and will provide an update in due course. The study results will also be submitted for publication in a peer-reviewed journal.
Ongoing ACTIV-2 trial
SNG001 is being investigated for possible use in COVID-19 patients at home as part of the US National Institute of Health`s ACTIV-2 trial. In October 2021 Synairgen announced that SNG001 had been recommended to advance from Phase 2 into Phase 3 in this trial in mild to moderate COVID-19 patients.
Tom Wilkinson, Chief Investigator and Professor of Respiratory Medicine, University of Southampton, said: "The results of the SPRINTER study confirm that inhaled interferon beta can be safely administered to hospitalised patients with COVID-19. The primary study endpoints of time to hospital discharge and time to recovery have not shown a clear treatment benefit. However, there is an important trend in favour of SNG001 in the secondary outcome progression to severe disease and death, which aligns to the findings of the earlier Phase 2 study. Better treatments for severe disease are still desperately needed but what`s clear is that improvements in outcomes driven by vaccination and the evolution in standards of care mean that larger studies are required to definitively explore SNG001`s impact on mortality and severe disease. With the strong safety profile that this treatment now has shown, it is appropriate for it to be considered by the large platform studies to confirm efficacy signals in severe COVID-19."
The Company`s current cash balances as of today are in excess of £25 million.
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No. 596/2014 (`MAR`).
About SPRINTER (SG018) trial
The SPRINTER trial (SG018; NCT04732949) is a global Phase 3, randomised, placebo-controlled, double-blind, multi-site clinical trial assessing the efficacy and safety of inhaled SNG001 on top of standard of care for the treatment of adults hospitalised due to COVID-19 requiring treatment with supplemental oxygen by mask or nasal prongs. Patients requiring high-flow nasal oxygen therapy, non-invasive ventilation, or endotracheal intubation (invasive ventilation) at randomisation were excluded. COVID-19 was confirmed using a validated molecular test for the presence of the SARS-CoV-2 virus.
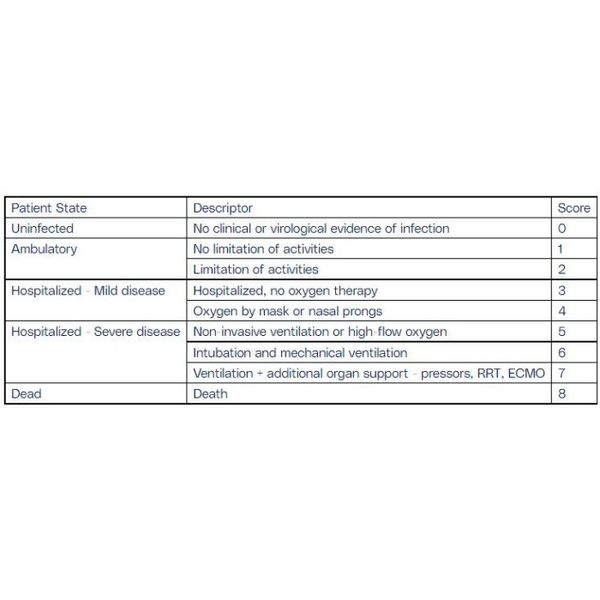
The primary efficacy analysis was performed in the intention-to-treat population (all randomised patients) by evaluating the change in clinical condition using the WHO 9-point Ordinal Scale for Clinical Improvement (OSCI; See Table 1 below) out to Day 35. Participants will be followed out to Day 90 to allow the assessment of long-COVID symptoms. The trial had two primary endpoints, evaluated using Cox proportional hazards modelling:
- Time to hospital discharge through Day 28, defined by the OSCI score of 2 or below, with no rebound (readmission) at subsequent assessments; and
- Time to recovery to "no limitation of activities" through Day 28, where recovery is defined as the OSCI score of 1 or below, with no rebound at subsequent assessments.
Key secondary endpoints, analysed using logistic regression, were:
- Progression to severe disease or death, defined by the WHO OSCI score of 5 or above within 35 days of first dose;
- Progression to intubation or death, defined by the WHO OSCI score of 6 or above within 35 days of first dose; and
- Death within 35 days of first dose.
- The trial enrolled 623 patients, randomised (1:1) to treatment with inhaled SNG001 or placebo on top of standard of care at more than one hundred sites across 17 countries including Argentina, Belgium, Brazil, Colombia, France, Germany, India, Israel, Italy, Mexico, Netherlands, Portugal, Romania, Serbia, Spain, the United Kingdom and the United States.
- SNG001 (15.6MIU) or placebo (formulation buffer without interferon beta) were administered by patients in the hospital, and at home once discharged, once-daily for up to 14 days using the Aerogen Solo/Ultra nebuliser.
Table 1: WHO Ordinal Scale for Clinical Improvement (OSCI)
SNG001 is a pH-neutral formulation of interferon-beta (IFN-beta) for inhalation that is delivered directly into the lungs using a mesh nebuliser, currently being investigated as a potential host-directed antiviral treatment for COVID-19 patients.
The SARS-CoV-2 virus has been shown to suppress the production of IFN-beta, a naturally occurring protein that orchestrates the body`s antiviral defences, with the aim of evading host immune responses. By administering IFN-beta into the lungs, the aim is to correct this deficiency, potentially switching back on the lungs` antiviral pathways to clear the virus.
About SynairgenSynairgen is a specialist respiratory biotechnology company whose primary focus is developing its inhaled IFN-beta candidate (SNG001) for the treatment of COVID-19 and other severe viral lung infections. SNG001 has been granted Fast Track designation from the US Food and Drug Administration (FDA) and its Phase 3 SPRINTER trial was deemed an Urgent Public Health study by the UK`s National Institute for Health Research (NIHR). Founded by University of Southampton Professors Sir Stephen Holgate, Donna Davies, and Ratko Djukanovic in 2003, Synairgen is quoted on AIM (LSE: SNG).
