- Home
- Companies
- Alfa Chemistry
- Products
- Alfa - Alkenyl Fluorinated Building ...
Alfa - Alkenyl Fluorinated Building Blocks
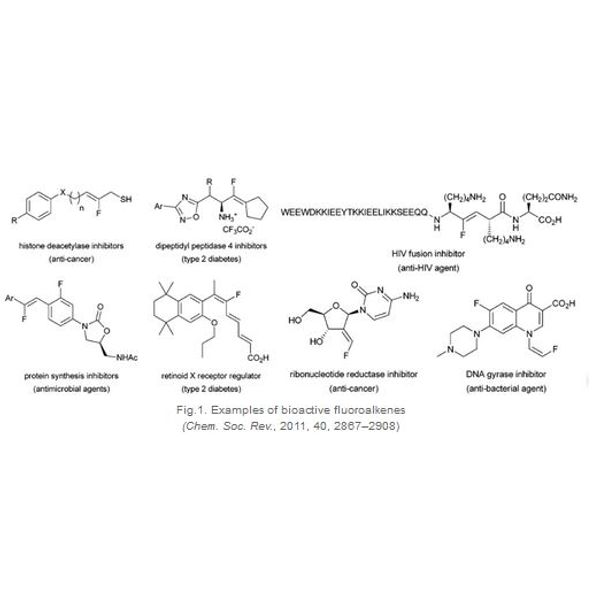
Fluorinated molecules occupy a significant place in pharmaceutical, agrochemical, and material sciences, due to the unique properties of the fluorine atom. The introduction of a fluorine atom can modulate the properties of a bioactive molecule, which may lead to changes in solubility, lipophilicity, metabolic stability, conformation, hydrogen-bonding ability and chemical reactivity. Within the various fluorinated compounds, fluorinated alkenyl is of particular interest. It is not surprising to find a number of bioactive compounds with various pharmacological activities (such as anticancer, antimicrobial, anti-HIV, anti-diabetic) bearing this motif (Fig 1).
Alkenyl fluorinated building blocks have potential applications in material sciences and synthetic organic chemistry where it can be used as a fluorinated synthon for further functionalization. Moreover, fluorinated alkenyl can be utilized in medicinal chemistry, as peptide bond isosteres, which open new opportunities in searching for new biologically active compounds.
- Olefination reactions: a-Fluoroacrylate derivatives are very useful fluorinated synthons which are used as monomers in the production of transparent films or in the fabrication of optical fibers. In 1990, Thenappan and Burton reported a reduction-olefination sequence to convert ethyl formate to 2-fluoroacrylate with good yields (Scheme 1).
- Elimination reactions: The elimination reaction is a common method to access a-substituted-a-fluoroalkenes. For the conversion of terminal alkenes into their fluorinated equivalents, the halofluorination reaction followed by elimination is widely used (Scheme 2).
- Electrophilic fluorination: Pacheco and Gouverneur have studied the reactivity of allenylmethylsilanes in the presence of Selectfluors in order to carry out an electrophilic fluorodesilylation for the preparation of fluorodienes, without use of a fluorinated building block (Scheme 4).