

- Home
- Companies
- atHeart Medical
- Products
- atHeart reSept - ASD Occluder Device

atHeart reSept - ASD Occluder Device
Defects vary in size. Some may close on their own early in life, while others require an intervention. Today, the vast majority are performed through minimally invasive catheter-based interventions. They consist in implanting an atrial septal occluder through a transcatheter procedure to close the defect. Current ASD occluder devices have dense metal frames that permanently clamp the septum. The long-term presence of metal in the heart may lead to potential complications and may limit future interventions that require crossing the interatrial septum.
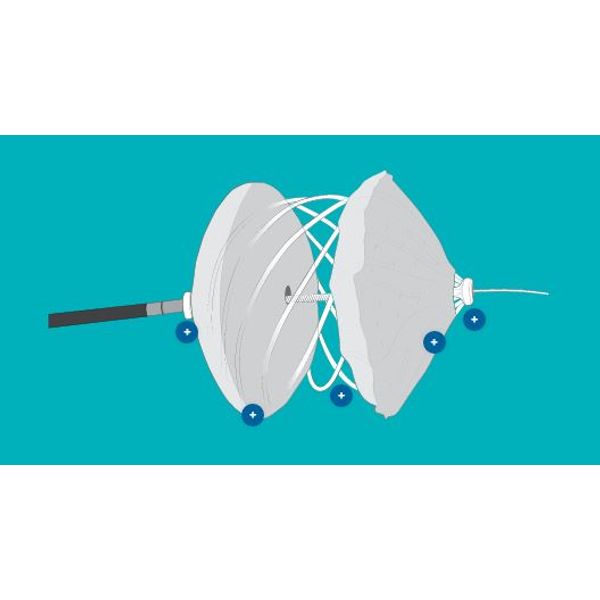
The reSept ASD Occluder is the first occluder with a metal-free, bioresorbable frame designed for the closure of atrial septal defects.
Delivered through a 12F sheath and over a standard guidewire, the low-profile device features bioresorbable filaments connecting two polyester fabric patches, which contain radiopaque markers.
The device is fully deployed with the guidewire in place, providing the opportunity to reattach and reposition when necessary.
After endothelialization, the filaments slowly resorb, with complete resorption demonstrated in-vitro at 24 months. The polyester fabric and the radiopaque markers remain, which may be useful for future transseptal procedure planning. Available in three sizes, the device supports closure of defects from 4-22mm.
ASCENT-ASD Investigational Device Exemption (IDE) Trial: atHeart Medical is enrolling patients in its pivotal IDE trial to demonstrate the safety and efficacy of the reSept™ ASD Occluder for treating clinically significant secundum ASD with a transcatheter approach as compared to pre-defined performance goals from other commercially available occluder devices. The prospective, single-arm, multi-site clinical investigation will enroll a total of up to 250 patients.
