- Home
- Companies
- Cerca Biotech
- Products
- CercaTest - Model Pre-eclampsia Vue - ...
CercaTest - Model Pre-eclampsia Vue - Assays
Disorders of pregnancy touch all parts of our world, access to rapid decision making tools can literally save the lives of both mothers and babies. Our first product for the management of pregnancy is the CercaTest™ Pre-eclampsia Vue™ assay. Using the well-established correlation between the excretion of misfolded proteins and the core causes of pre-eclampsia, we have developed a simple cost effective test that can be used in hospitals, in primary care and hopefully in time by patients themselves to check that signs and symptoms of pre-eclampsia do not become life threatening.
In 2014, Professor Irina Buhimschi then at Yale University first published her discovery of the presence of misfolded proteins in urine samples from pregnant women and their correlation with pre-eclampsia.
Congophilia (binding affinity of misfolded proteins to Congo red dye), a long-known property of misfolded proteins, was employed to detect misfolded proteins in urine samples from pregnant women. Diagnosis of pre-eclampsia based on Congophilia was later validated independently by researchers in the US, the UK, Israel, India, Mexico, and China.
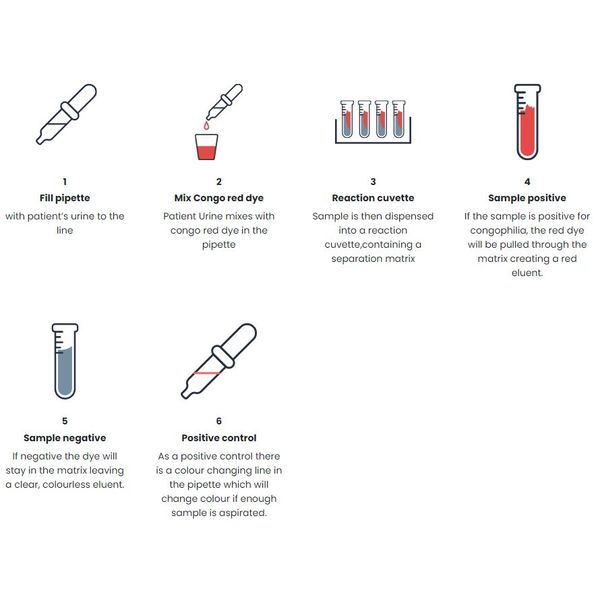
Several methods and devices have been developed to detect Congophilia and diagnose pre-eclampsia. Our CercaTest™ Pre-eclampsia VueTM, is an innovative device based on a novel technique that enables a simple and accurate point-of-care test of pre-eclampsia within 5 minutes.
Urine from pregnant women with pre-eclampsia typically contains aggregates of misfolded proteins, which can be selectively bound by Congo red dye (congophilia). When a mixture of Congo red dye and urine from a pregnant woman is loaded onto the separation matrix in a detection cuvette, the presence (positive) or absence (negative) of misfolded proteins can be determined based on the colour of eluate collected in the cuvette. A red eluate colour indicates a positive pre-eclampsia diagnosis, whereas a colourless eluate indicates a negative diagnosis.
Cerca Biotech is currently enrolling centres across Europe to carry out clinical evaluations of the CercaTest™ Pre-eclampsia Vue™ assay. If you or your centre would like to get involved please contact us to discuss further.
- Sensitivity: 74.3%
- Specificity: 98.4%
- NPV: 95.5%
- Accuracy: 94.7%
The CercaTest™ Pre-eclampsia Vue™ assay was tested in a prospective cohort of nearly 1000 patients across several centres in China. When compared to a diagnosis of Pre-Eclampsia using standard diagnostic confirmation, the above results were observed, paper pending.
12 Test per Kit, built in process controls
Rapid Urine based assay
3-5 ml sample
5 minute read time
Straight forward colour change assay
Simple to read