Lepu Medical Technology (Beijing) Co.,Ltd.
- Home
- Companies
- Lepu Medical Technology (Beijing) ...
- Products
- MemoCarna - Atrial Septal Defect (ASD) ...

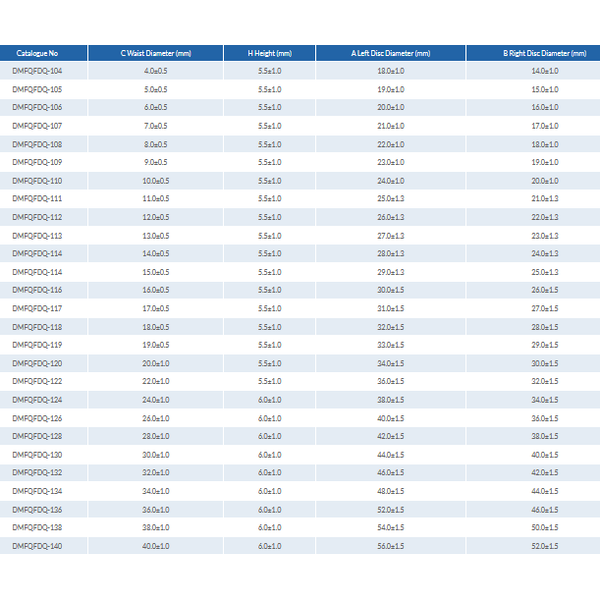
MemoCarna - Atrial Septal Defect (ASD) Occluder
Oxide film surface treatment solution: Reduce the amount of metal implantation. Easy to release and recycle. Umbrella surface is flatter. Less nickel ions precipitated. Excellent push performance.
Most popular related searches
atrial septal
congenital heart disease
coronary sinus
pulmonary vein
cardiac disease
blood flow
medical device
implant
thrombus
medical titanium
- MemoCarna® Atrial Septal Defect (ASD) Occluder with single hub
- The product is composed of a nickel titanium alloy stent, a stainless steel bushing and a polyester fiber membrane.
- The stent is made of medical nickel-titanium memory alloy wires and filled with the polyester fiber membrane, to be applied for the atrial septal defect.
- A stainless steel screw bushing used for fixing the nickel-titanium alloy wire on one end thereof, and the nut of the steel screw bushing may match with the screw of the head end of the conveyor pushing rod.
- Suitablefor the secundum left-to-right shunt atrial septal defect (ASD) with the ASD diameter of ≥ 5mm and ≤ 36mm and the increased right ventricular volume overload;
- The defect edge is ≥ 5mm distanced from the coronary sinus, the superior or inferior vena cava and the pulmonary vein, and is ≥ 7mm distanced from atrioventricular valve.
- Other cardiac abnormalities required for the surgery are not combined.
- Well coordinated with delivery system, multi withdraw, release and antifatigue performance.
- After 3 months implantation, CARNA occluder positioned well, without residual shunt, smooth two disks surface without thrombus or neoplasms.
- Adopt updated structure braiding technology, with advanced heat treatment technique,
- guarantee additional advantages:
- Effectively decreased nitinol ions release into human body.
- Accelerated endothelialization process, minimize thrombus risk.
- Single hub design reduce metal implantation and device-driven thrombus risk.