

- Home
- Companies
- Revotek Co., Ltd.
- Products
- Revotek - Bio-Vascular Grafts
Revotek - Bio-Vascular Grafts
It is estimated by the World Health Organization that the current global prevalence of advanced vascular disease is ~175mm people and increasing. One of the most common clinical interventions to treat advanced vascular disease is the replacement of stenotic or occluded arteries with vascular grafts. This is often accomplished utilizing autologous veins/arteries or through synthetic conduits composed of materials such as polyester or expanded polytetrafluoroethylene (ePTFE).
Synthetic vascular grafts have been rapidly growing in favor over the use of autologous grafts (i.e. natural vessels taken from the patient’s own body) as the use of the patient’s own vasculature is often restricted by the general unavailability of suitable vascular tissue in patients with advanced disease states and due to the additional complications arising from multi-stage surgeries to explant a healthy vessel from one location and implant in another.
Due to the growing demand for synthetics, the synthetic vascular market is expected to reach $3.4 billion a year in global sales by 2022. Although preferred over autologous vessels, the efficacy of the available synthetic solutions on the market is unacceptably low - resulting in unnecessary long term costs and post-operation complications for patients.
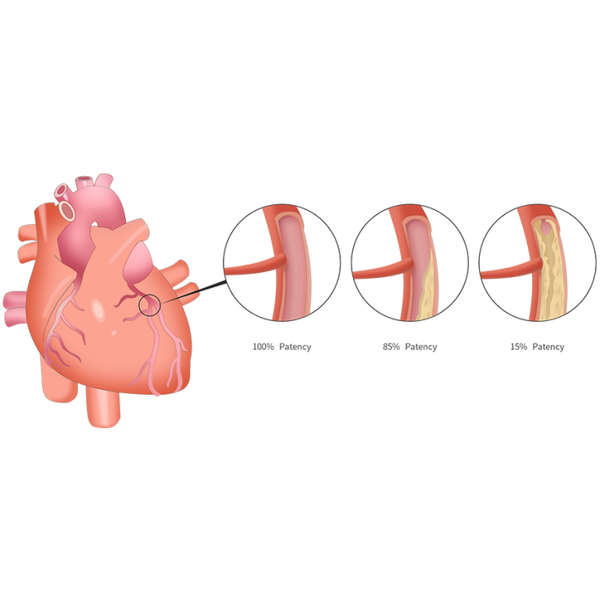
Patency measures the unobstructed level of flow through a vascular structure. It is the standard benchmark for evaluating the efficacy of a product. 100% patency represents a healthy, fully functioning vascular structure. 15% patency represents one that is 85% obstructed.
The common, unresolved issue of these prosthetic grafts is thrombosis, restenosis, compliance mismatch, low long-term patency, long-term anticoagulation therapy and limited service life due to the lack of or improper formation of a smooth endothelium.
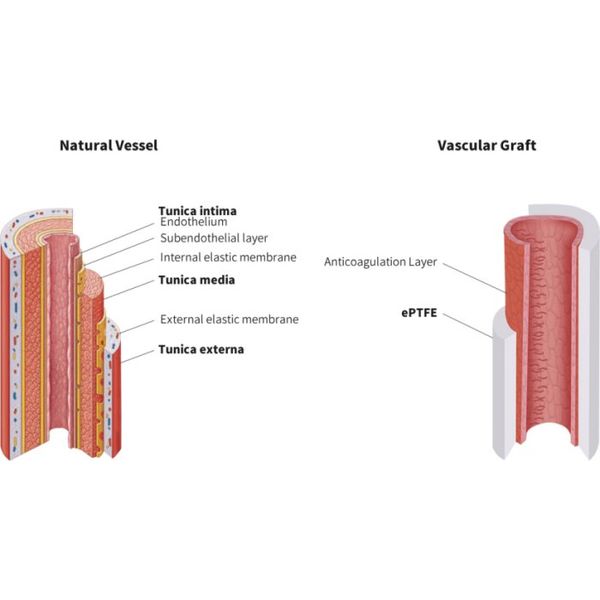
A product that enables the formation of a completely smooth endothelial layer would resolve many of the challenges facing currently available synthetic vascular grafts on the market. Such a solution would vastly improve patient outcomes and be likely to quickly gain favor as the preferred product within the industry.
As detailed below, Revotek’s 3D-printed bio-vascular grafts allow for the formation of a completely smooth endothelial layer within weeks of implantation.
According to data from the NIH, 1 year patency rates for PTFE-based vascular grafts are as low as 51% for operations such as coronary arterial bypass grafts. A product that could allow the formation of a completely smooth endothelial layer would be vastly superior to the alternatives available on the market today.
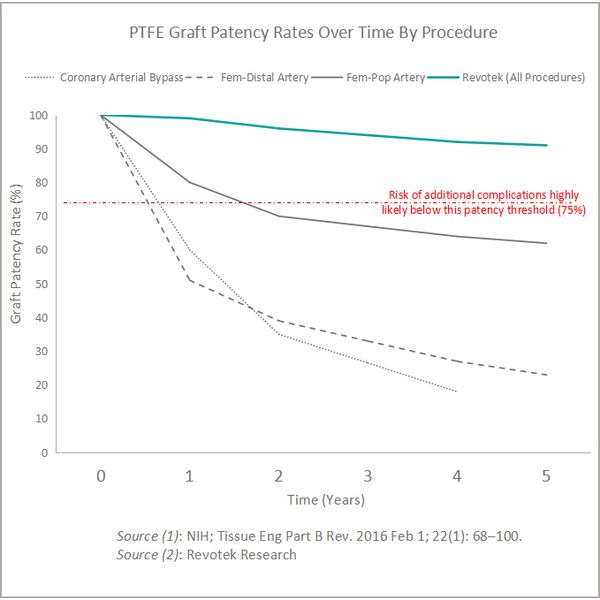
In H2 2016 Revotek`s scientific team used its patented processes and technologies to print and graft blood vessels that were fully accepted in 100% of the living hosts that were part of the trial. The printed vessels all formed a fully functioning, smooth endothelial layer within 3 weeks of being implanted into the hosts.
1.Modeling
Revotek‘s RevoCloud™ technology converts 2D scans (e.g.CT) into 3D representational models used in fabrication
2.Extraction5 grams adipose tissue extracted from host and added to a proprietary culture
3.PreparationCells are encased with proprietary Biosynsphere™ preparation
4.FabricationScaffold-free,undifferentiated vascular structures printed using Revotek’s T-Series™ 3D Bio Printer
5.GraftingBlood vessels implanted as an interpositional graft
6.AcceptanceWithin 4 weeks the grafted vessel self-differentiates and becomes indistinguishable form naturaally comparable surrounding vessels
