

GE HealthCare
- Home
- Companies
- GE HealthCare
- Products
- GE HealthCare - Model Invenia ABUS 2.0 ...
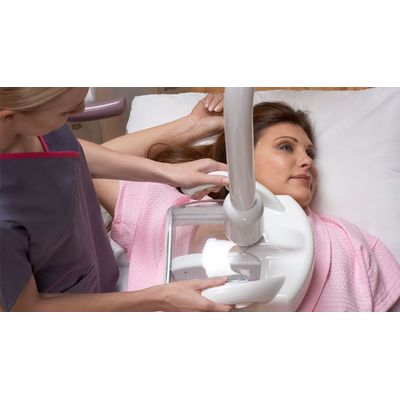
GE HealthCare - Model Invenia ABUS 2.0 -Breast Ultrasound Solutions & Systems
FromGE HealthCare
The Invenia ABUS 2.0 is designed as an FDA-approved ultrasound supplemental screening technology, aiming to improve cancer detection in women with dense breast tissue. It offers a 35.7% increase in detection over traditional mammography, thanks to its superior image quality and advanced interpretation tools. The system integrates AI assistance to enhance clinical confidence by detecting and characterizing breast lesions, thus aiding healthcare practitioners in identifying small, invasive cancers that might go unnoticed with conventional imaging. Additionally, the system is built for user-friendly operations, allowing for comprehensive lesion assessment through labeling, measuring, and comparison tools, including 2D shear wave elastography for tissue elasticity assessment. The Invenia ABUS 2.0 stands as an imperative tool in providing personalized and comprehensive breast cancer screening and diagnosis.Most popular related searches
breast ultrasound
breast tissue
breast cancer detection
breast cancer monitoring
cancer detection
ultrasound system
breast cancer
healthcare practitioner
breast screening
cancer screening
Clinical evidence is growing about the effectiveness of ultrasound for finding small, node-negative, invasive cancers that may be missed by mammography1. Patient-friendly Invenia™ ABUS 2.0 is the first FDA-approved ultrasound supplemental screening technology that is specifically designed for detecting cancer in dense breast tissue.


