

- Home
- Companies
- Ivy Biomedical Systems, Inc
- Products
- Ivy Biomedical - Model 7800 - Cardiac ...
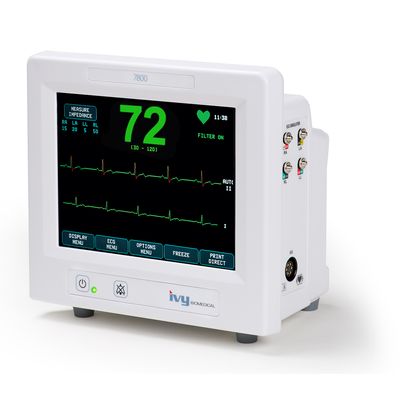
Ivy Biomedical - Model 7800 -Cardiac Trigger Monitor
The Model 7800 is Ivy Biomedical System’s 5th generation cardiac trigger monitor used in applications requiring precision ECG R-wave synchronization for gating host systems on and off. The Model 7800 incorporates an intuitive, menu-driven, touch-screen user interface, simplifying operation for clinicians and technicians.
Applications
The Model 7800 is a favorite for use in cardiovascular diagnostic imaging studies, especially in computed tomography (CT) applications.
Worry Free Operation
Acquiring reliable and consistent ECG triggers can often be a challenging task, and selecting the right ECG vector can be problematic. The Model 7800 uses a 4-lead ECG configuration in order to provide automatic lead selection capability. This feature monitors each ECG vector simultaneously, and automatically selects the best ECG lead for triggering, thereby delivering reliable triggering even under challenging or changing patient conditions. Red markers on the ECG waveform indicate when and where triggering occurred for visual verification and assurance. In addition, an electrode impedance measurement function enables quick verification of proper electrode/skin preparation prior to patient scans.
Key Features
The Model 7800 offers a large 8” diagonal color TFT display, ECG simulator, heart rate limit alarms, electrode impedance check, X-ray on signal input, ECG waveform export, Ethernet and RS-232 communication interface, multiple factory configured trigger output pulse width and amplitude options, optional integrated strip chart recorder, optional roll stand mount, and more.
System Integration
Several system integration features are provided to enable streamlined and automated operation including ECG waveform output to both the gantry display and operator’s console, X-ray on signal input for ECG waveform file capture for each patient scan, and Ethernet communication for remote command and control.
Precision Performance
The Model 7800 offers trigger pulse output performance with less than 2ms of delay, and ±75µs of dither (accuracy), ensuring consistent and reliable triggering.
Regulatory & Compliance
All Ivy Biomedical products comply with global standards for safety, EMC, quality management, and environmental requirements.
- Trigger Lead: LI, LII, LIII, and AUTO - menu selectable.
- Second Lead: LI, LII and LIII – menu selectable.
- Patient Cable: 4-lead patient cable with 6-Pin AAMI standard connector.
- Isolation: Isolated from ground related circuits by >4 kV rms, 5.5 kV peak
- Electrode Impedance MeasurementTechnique: 10 Hz ac signal < 10 uA rms
- Range: 200kO per lead
- Accuracy: ±3% ±1kO
- Recommended Electrode: 10% Chloride sponge type (Ivy P/N: 590436)
- CardiotachRange: 10 to 350 BPM (Pediatric / Neonate)
- 10 to 300 BPM (Adult)
- Accuracy: ±1% ±1 BPM
- Resolution: 1 BPM
- Sensitivity: 300 µV peak
- Tall T Wave Rejection: Rejects T waves = 1.2 * R-wave
- Pacer Pulse RejectionWidth: 0.1 to 2 ms at ±2 to ±700 mV
- AlarmsHigh Rate: 15 to 250 BPM in 5 BPM increments
- Low Rate: 10 to 245 BPM in 5 BPM increments
- Asystole: R to R interval >6 seconds
- Lead Off: Detached lead or offset potential >0.5 V
- Test ModeInternal: ECG 1 mV/100 ms referred to input @ 70 BPM
- Simulator: ECG waveform amplitude: 1mV
- Simulator Range: 10 – 250 BPM.
- Simulator Rate: Variable rate in steps of 30, 60, 90, 120, 150, 180, 210 and 240 BPM. Manually adjustable in increments of 1 BPM.
- DisplayActive Matrix TFT Color Touch Screen LCD (640x480)
- Screen Size: 17.09cm x 12.82cm, 21.36cm (8.4") diagonal
- MechanicalSize: Height: 8.72in. (22.14cm)
- Width: 9.25in. (23.50cm)
- Depth: 6.10in. (15.49cm)
- Weight: 5.6 lbs. (2.54kg)
- Writing Method: Direct Thermal
- Synchronized Output (Trigger)Test input signal at ECG leads: Conditions: ½ sine wave, 60ms width, 1mV amplitude, 1 pulse/second
- Output Trigger Delay: < 10 ms
- R to R Trigger Accuracy: ±75 µs typical @ 1 mV input
- Pulse width: 100ms
- Pulse amplitude: 0 to +5V
- Output Impedance:
- Operating EnvironmentTemperature Range: 15°C to 40°C
- Relative Humidity: 0-90% non-condensing
- Altitude: -100 meters to +3,000 meters
- Atmospheric Pressure: 500-1060 mbar
- Protection against ingress of fluids: IPX1 – Protection against vertically falling drops of water
- Storage EnvironmentTemperature Range: -20°C to 49°C
- Relative Humidity: 0-100%
- Altitude: -100 meters to +14,000 meters
- Power RequirementsVoltage Input: 100-120V~; 200-230V~
- Line Frequency: 50/60 Hz
- Fuses Type and Rating: T.5A, 250V (Metric 5x20 mm)
- Maximum ac Power
- Consumption: 45 VA
- Power Recovery: Automatic, if power is restored within 30 seconds
- CommunicationsEthernet 10Base-T, IEEE 802.3 (2 Channels)
- RS-232
- Data StorageUSB Memory Stick
- AAMI Cardiac Monitor Standard EC-13
- UL60601-1
- CAN/CSA C22.2 No 601.1-M90
- CDN MDR (CMDCAS)
- IEC 60601-2-27
- MDD.93/42/EEC
- CE 0413
- ISO 13485:2003
- FDA/CGMP
