

- Home
- Companies
- Premier Biotech, Inc.
- Products
- Premier Biotech - CLIA-Waived Rapid ...
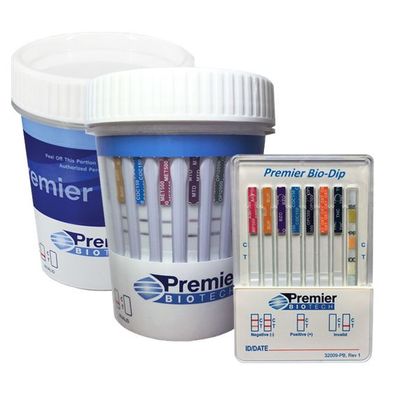
Premier Biotech - CLIA-Waived Rapid Urine Drug Testing Products
Premier Biotech’s CLIA-Waived (Clinical Laboratory Improvement Amendments) multi-drug integrated cup and dip products offer the ability to test for 5-14 drugs. CLIA-Waived test products are cleared by the FDA for over-the-counter (OTC) use, suited for workplace and clinical drug testing programs and approved for waiver under the CLIA criteria.
As an R&D driven organization, our product lines and services continue to evolve as we introduce new technology to the market. We understand the unique needs of our customers, from Workplace to Clinical based testing programs, our CLIA-Waived products and services can ensure your testing program is operating smoothly. Our rapid, integrated screening products are distinguished by their accuracy, fast results and ease of use.
- Integrated, multi-strip panel offerings
- Highly accurate, sensitive, and specific
- One drug per strip ensuring clear results
- Easy to use, single step procedure
- Easy to read results with strong line intensity
- Fast results in minutes
- AMP-Amphetamines
- BAR-Barbiturates
- BUP-Buprenorphine
- BZO-Benzodiazepines
- COC-Cocaine
- EDDP-Methadone Metabolite
- MDMA-Ecstasy
- MET-Methamphetamine
- MTD-Methadone
- OPI-Opiates
- OXY-Oxycodone
- PCP-Phencyclidine
- TCA-Tricyclic Antidepressants
- THC-Marijuana
