

- Home
- Companies
- NeuroOne Medical Technologies ...
- Products
- NeuroOne - Model Evo - Cortical ...
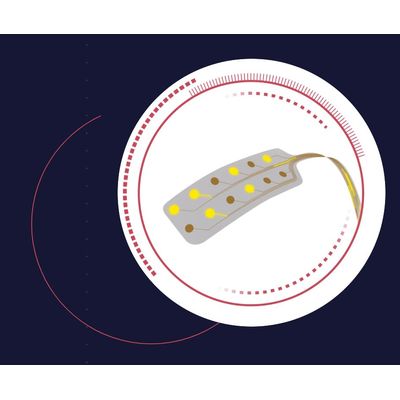
NeuroOne - Model Evo - Cortical Electrode
Cortical or subdural electrodes are used in electrocorticography (ECoG) or intracranial electroencephalography (iEEG) surgeries to monitor, record and stimulate the surface of the brain for up to 30 days. Our Evo cortical electrode portfolio consists of various contact configurations of strip and grid electrodes. (Rx only)
In comparison to currently available technologies, our electrodes are manufactured with polyimide thin film. Our electrodes are designed to reduce trauma to the brain by allowing a less invasive implant due to the flexibility, thinness and reduced weight of the electrode.
Furthermore, the potential to significantly increase the resolution of brain recordings may enable the usage of powerful computing techniques, such as machine learning and artificial intelligence.
Number of Contacts: 2 - 16
Contact Spacing: 10mm
Contact Size: 3mm
Electrode Contact Material: Platinum
Electrode Substrate Material: Polyimide
Thickness: 0.08mm
Weight: 0.05 grams
Tail Length: 12.70 inches
Disposable Cables: Proprietary cable assembly for the Evo electrode with a zero force connection box and standard DIN42802 touch-proof connectors compatible with standard headboxes.
See the Instructions for Use for additional information (e.g. Description, Indications for Use, Contraindications, Warnings, Adverse Events, etc.)
Decreased Immunological Response - The Evo Electrode is over 7 times thinner than a silicone electrode. The flexibility and reduced volume should reduce pain and edema. 2
The Evo’s polyimide substrate has properties like increased flexibility, reduced volume, and decreased immunological response, which should reduce signal artifacts. 3
Single Tail Design - The single thin tail design allows the implanted electrode tail to be tunneled through one incision which should reduce infection risk and procedure time. 1,2
Reduced Cable Management - A disposable cable assembly is sent with each Evo Electrode as an electrode kit, removing the need to source the correct cables for each electrode being used in surgery. Also, hospital resources are freed from the management of sterilizing and storing individual electrode cable assemblies.
