


Getein Biomedical - COVID-19 SARS-CoV-2 Antigen Rapid Test Kit (Immunofluorescence Assay)
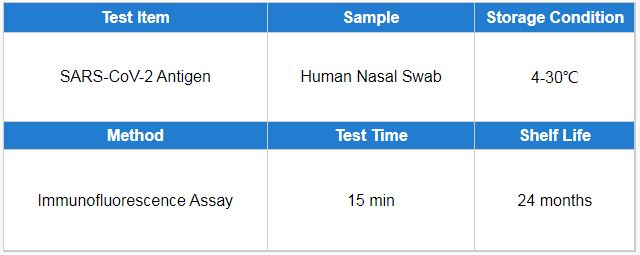
Test Item: SARS-CoV-2 Antigen. Sample Type: Human nasal swab. Test Time: 15 min. Methodology: Immunofluorescence Assay.
Intended Use
SARS-CoV-2 Antigen Fast Test Kit (Immunofluorescence Assay) is intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in human nasal swab samples from patients suspected of COVID-19 infection by a healthcare provider.
About SARS-CoV-2
The novel coronaviruses belong to the β genus. SARS-CoV-2 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Contents
1. For Getein1100
Package specifications: 25 tests/box
- Getein SARS-CoV-2 antigen test card in a sealed pouch with desiccant
- Sample extraction solution: 25 tubes/box
- Sampling swab: 25 pieces/box
- User manual: 1 piece/box
- SD card: 1 piece/box
2.For Getein1600
Package specifications: 2×24 tests/kit, 2×48 tests/kit
- Sealed cartridge with 24/48 Getein SARS-CoV-2 antigen test cards
- User manual: 1 piece/box
Materials required for Getein1600:
- Sample extraction solution: 1 bottle/box
- Box with pipette tips: 96 tips/box
- Mixing plate: 1 piece/box
- Sampling swab: 48 pieces/box, 96 pieces/box
Note: Do not mix or interchange different batches of kits.
Getein1100 Immunofluorescence Quantitative Analyzer
Getein1600 Immunofluorescence Quantitative Analyzer
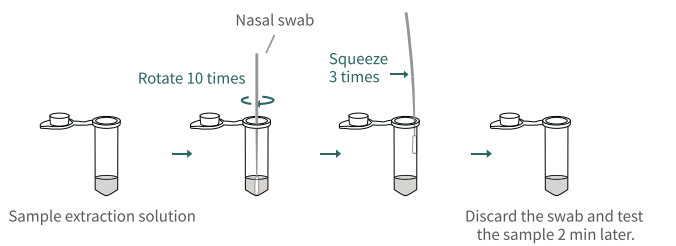
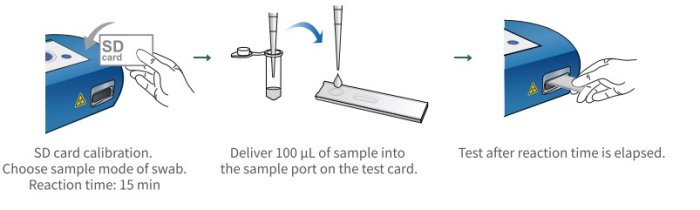
- This test is an aid in the diagnosis of patients with suspected SARS-CoV-2 infection in conjunction with clinical presentation and the results of other laboratory tests.
- This test is only intended for professional and laboratory use, not for home testing.
- Results from the test should not be used as the sole basis for diagnosis and exclusion of SARS-CoV-2 infection.
- As it is a novel disease diagnosis of which are being explored, please refer to the latest guidelines for diagnosis and treatment of COVID-19.
