- Home
- Companies
- Wuhan HealthCare Biotechnology Co., ...
- Products
- Covid-19 Standard, Alpha & Beta ...

Covid-19 Standard, Alpha & Beta Variants Test Kit
This kit is used for the in vitro qualitative detection of suspected cases of pneumonia caused by New Coronavirus infection, patients with suspected clusters, other patients who need to be diagnosed or differentially diagnosed with the New Coronavirus infection, and nasopharyngeal swabs, oropharyngeal swabs, and bronchoalveolar lavage fluid samples of patients with mutation beads for the New Coronavirus ORF1ab and N gene, S gene N501Y mutation and S gene E484K mutation sites.
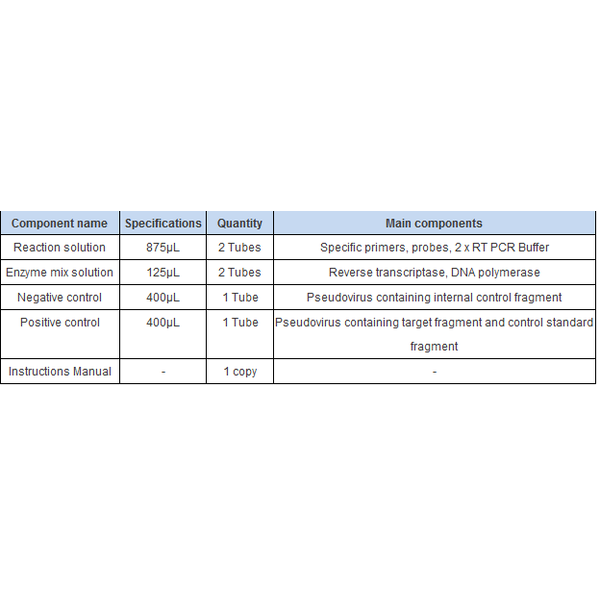
Packing Specifications100 Tests/Box.
Detection Principle
This kit is designed with two pairs of specific primers and Taqman probes for the New Coronavirus ORF1ab and the conservative region of the N gene sequence encoding the nucleocapsid protein of the new coronavirus to diagnose or differentially diagnose whether the tested patient is infected with the new coronavirus, the British B.1.1.7 strain-specific N501Y mutation site and the South African B.1.351 strain-specific E484K mutation sites.
The PCR reaction system contains primers and probes for internal standard. The process of sample collection, extraction and reaction is monitored by detecting internal standard to avoid false negative results.
Storage Conditions & Validity]
1. The kit should be stored frozen at -20?±5? and protected from light; the expiration date is 6 months; the production date and expiration date are shown in the outer packaging box.
2. Avoid repeated freezing and thawing of the kit and the number of freezing and thawing shall not exceed 7 times.
3. After opening, the bottles should be stored at -20?±5? and protected from light. The number of bottles opening times should not exceed 7 times, which will not affect the use within the validity period.
Applicable Instruments
- This kit has been validated on ABI7500 quantitative fluorescence PCR instrument.
- For other models not listed, relevant experiments have not been performed or completed for this kit. If users need to use this type of instrument platform to carry out the detection of this reagent, please contact our Technical Department at cs@healthcare-bio.com for relevant support.
NB:The other devices can include quantitative fluorescence PCR platforms with FAM, VIC, ROX and Cy5 channels.
Sample Requirements
Nasopharyngeal swabs, oropharyngeal swabs and other methods are used to obtain samples, and it is recommended to use commercial virus sampling kits for sample collection devices. The specific operation method is as follows:
i) Nasopharyngeal swabs: Nasopharyngeal swab: The operator gently rotates the sterile swab to the left and right at the angle parallel to the upper jaw and inserts it from one nostril to the nasal palate in the nasal passage. Generally, the swab stays when there is resistance to the insertion Rotate slowly to exit after 2-3s.
ii) Oropharyngeal swab: The operator holds the tongue depressor and presses the back root of the patient`s tongue in one hand, and holds the root of the sterile swab in the other hand, and then quickly and firmly apply the sterile swab to the left and right sides and the swab head. Scrape the secretions on both sides of the tonsils and the back wall of the pharynx.
iii) Place the nasopharyngeal swab or oropharyngeal swab into the centrifuge tube containing the preservation solution for later use.
2. Alveolar lavage fluid sample: Use a sterile syringe to extract the sample and place it in a centrifuge tube for examination.
3. Cross-contamination between samples should be avoided.
4. Samples should be tested in time after collection, or stored at -20±5? for testing, and kept below -70? for long-term storage.
Testing Method
1. Reagent preparation: (Reagent preparation area)
In each PCR reaction, both the positive control and the negative control are tested simultaneously, and each sample needs to be tested for the ORF1ab region, N gene, S gene N501Y mutation, S gene E484K mutation and internal standard.
1) Remove the kit from the refrigerator, equilibrate at room temperature, fully dethaw the components, vortex to mix and then centrifuge instantly.
2) Calculate the number of reactions required for the current experiment n (n = number of samples + negative control + positive control), and mix the reagents according to the preparation method of the reaction system in Table 1 and then divide into 20μL/well PCR reaction tube. Transfer the PCR reaction tube to the sample preparation area, and put the remaining reagents back to -20?±5? and keep away from light.