

- Home
- Companies
- Hemostemix Inc.
- Products
- Hemostemix - Creating Personalized ...

Hemostemix - Creating Personalized Regenerative Cell Therapies Technology
Hemostemix’s technology uses a patient’s own cells to treat that patient’s disease. The cornerstone of this autologous technology is a novel cell population within the blood called the synergetic cell population (SCP). The synergetic cell population, which can be collected from a simple blood draw, consists of progenitor and other supporting cells that are being developed for the treatment of ischemic diseases. Hemostemix’s proprietary technology includes methods for collecting the synergetic cell population and manufacturing (isolation, enrichment and differentiation) a personalized regenerative therapy that can be administered to a patient within 7 days of the initial cell collection.
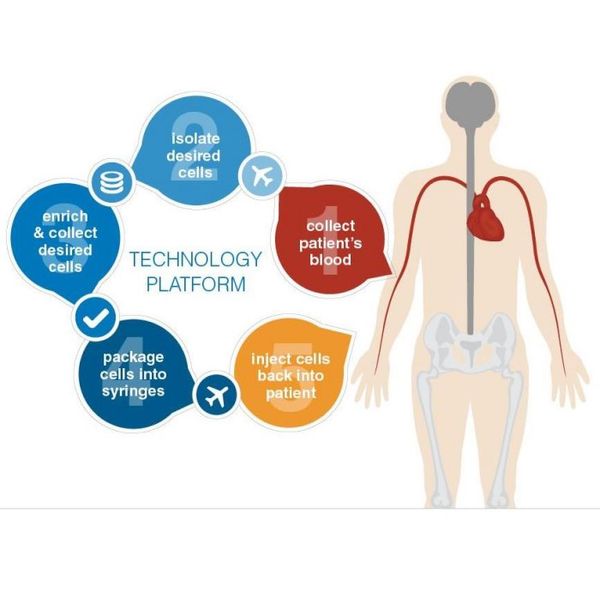
Hemostemix’s proprietary technology is protected by an extensive portfolio of issued and pending patents throughout the United States, Canada, Europe, Japan and China related to the collection, processing and manufacture of therapies from a cell population found in peripheral blood. Our proprietary technology is based on more than 10 years of clinical data demonstrating the ability of our autologous cell product to regenerate diseased and damaged tissue, which has the potential to generate therapies for a broad range of ischemic diseases such as critical limb ischemia, peripheral artery disease, congestive heart failure and other vascular diseases. Hemostemix develops its cell therapy products from a patient’s own blood which is a relatively low risk, cost effective and non-invasive source of therapeutic cells. The Company has over 91 patents and patent applications in more than 25 jurisdictions.
The Company’s patent portfolio covers five patent families which include:
- Regulating stem cells
- Regulating stem cells
- In vitro techniques for use with stem cells
- Production from blood of cells of neural lineage
- Automated cell therapy
ACP-01, which is currently in an international, multi-center Phase 2 clinical trial, has the potential to non-invasively treat patients with severe critical limb ischemia. Treatment with ACP-01 involves extracting and enriching the desired cell population from patient’s blood, and then injecting the modified cell population back into the patient to form new blood vessels in dying tissue.
- Self-donor: Uses patient’s own cells, no immune rejection, no observed safety issues
- Simple: Cell harvest via blood draw
- Quick: 7 days from draw to administration to patient
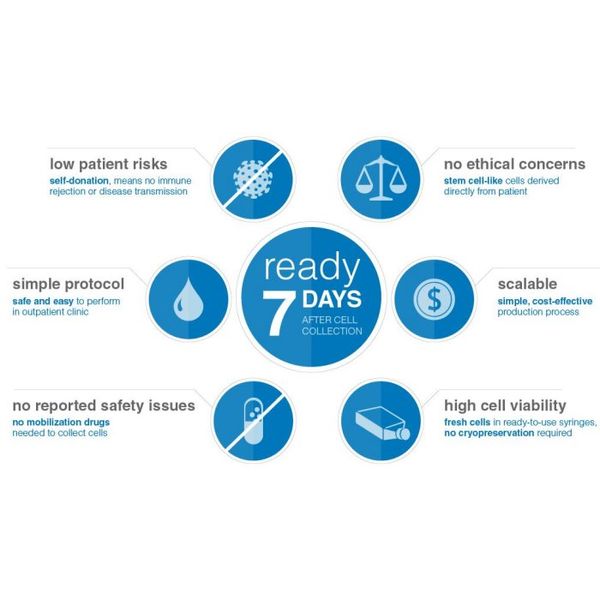
Treatment Ready within 7 Days
Following a blood draw, the subject’s blood is fractionated into its components. The stem cells are removed, cultured in the subject’s own blood serum, differentiated into angiogenic cell precursors, and returned to the subject in seven days. The final product is transported to the treating physician in a sterile ready-to-use syringe, which is contained in a temperature controlled environment that provides a stable product for 48-hours.
