

- Home
- Companies
- NeuroSigma, Inc.
- Products
- Monarch - eTNS System for Pediatric ...
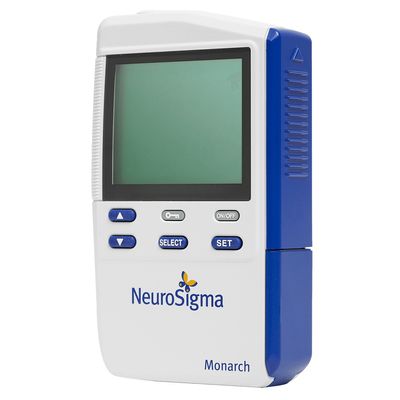
Monarch - eTNS System for Pediatric ADHD
Attention deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed neurological conditions in school-age children that often persists into adulthood. Parents and caregivers looking for alternatives to ADHD medication have a new treatment option: The Monarch eTNS (external Trigeminal Nerve Stimulation) System by NeuroSigma.
The Monarch eTNS System is now available to patients and physicians through NeuroSigma’s Early Access Program. There are two ways to obtain a device.
If you would like to work with your existing doctor or healthcare professional, simply download our PATIENT INFORMATION FORM. This download contains the Monarch eTNS prescription form for you to share with your healthcare professional and step-by-step instructions on how to get the device.
You may also use our PHYSICIAN FINDER PAGE to locate one of our Early Access Program centers familiar with the Monarch eTNS System.
In the United States, the Monarch is indicated for the treatment of pediatric ADHD as a standalone therapy (monotherapy) in patients ages 7 through 12 years old who are not currently taking prescription ADHD medications. The device is available by prescription only and is intended to be used in the home under the supervision of a caregiver during periods of sleep.
The most common side effects observed with eTNS use are: drowsiness, an increase in appetite, trouble sleeping, teeth clenching, headache and fatigue. No serious adverse events have been associated with use of the device. Please consult the instructions for use for additional information.
The Monarch eTNS System includes a cellphone-sized device that sends a low-level electrical pulse through a wire to a small patch adhered to the patient`s forehead. The therapeutic pulses stimulate the branches of the trigeminal nerve, which activates the neural pathway to other parts of the brain thought to be involved in ADHD. Neuroimaging studies have shown that eTNS increases activity in brain regions that are known to be important in regulating attention, emotion, and behavior.
The Monarch eTNS device should be used in the home under the supervision of a caregiver during periods of sleep. Each night at bedtime, the caregiver places the adhesive patch on the patient`s forehead, just above the eyebrows. Patients describe the stimulation as a tingling sensation on the skin. Clinical trials suggest that a response to eTNS may take up to 4 weeks to become evident. The performance of the device was assessed compared to a sham (placebo) device based on improvement in ADHD-RS (an assessment for ADHD symptoms) after 4 weeks. The results of the main clinical study for the device showed that patients who were treated with the Monarch eTNS improved significantly more than patients who were treated with a sham (placebo) device. More information on the Monarch eTNS clinical studies is available in the device user manual. Patients should consult with their healthcare professional after 4 weeks of use to assess treatment effects.
NeuroSigma is pioneering the use of trigeminal nerve stimulation (TNS) neuromodulation therapies for a variety of neurological and neuropsychiatric disorders, including ADHD. Our research efforts are led by an experienced team of doctors, scientists, and engineers. Use of TNS as a therapeutic platform is supported by over a decade of both basic science and clinical studies, which include:
The first double-blind randomized controlled trial of external Trigeminal Nerve Stimulation (eTNS) for pediatric ADHD included 62 children ages 8 to 12 to use eTNS as monotherapy for 4 weeks. The trial demonstrated statistically significant improvements in symptoms of ADHD as measured by the ADHD-RS-IV scale among children randomized to the treatment group as compared to those in the sham group. The trial also found significant changes in quantitative EEG recordings among subjects randomized to the treatment group as compared to those in sham. Results were published in the Journal of the American Academy of Child and Adolescent Psychiatry in 2019.
This 8-week open trial included 24 participants ages 7 to 14 with ADHD who had TNS administered nightly during sleep. Participants were assessed weekly with parent- and physician-completed measures of ADHD symptoms and executive functioning as well as treatment compliance, adverse events, and side effects. Computerized tests of cognitive functioning were administered at baseline and weeks 4 and 8. Results were published in 2015 in the peer-reviewed medical journal Brain Stimulation.
