


ResistancePlus - Model GC -Gonorrhoea and Ciprofloxacin Susceptibility
Single well qPCR test for detection of Neisseria gonorrhoeae and markers linked to ciprofloxacin susceptibility validated on a range of specimens and collection vessels. Antibiotic resistance in gonorrhoea infections is a global concern. There are limited effective treatment options and novel antibiotics are not readily available. Smarter and more directed use of antibiotics can be achieved through Resistance Guided Therapy. Global surveillance data indicate 50-70% of gonorrhoea infections remain susceptible to ciprofloxacin. Guidelines for gonorrhoea management call to preserve ceftriaxone and recommend use of ciprofloxacin when susceptibility information is available. Azithromycin resistance is rising, compromising dual-therapy options.
Streamline your workflow and increase productivity for rapid, routine diagnostics. Full automation options including sample and qPCR set-up, through to validated software solutions for automated result calling and simple data processing.
- Validated on a range of specimen types and collection devices
- Unique triple target solution, ideal for confirming GC positives or for upfront testing with simultaneous resistance status
SpeeDx analysis software included in contract pricing is license free and installed on a high security and GDPR compliant platform:
- audit trails
- user traceability
- LIS connectivity
- QA and batch management
Increase capacity of your existing qPCR instrumentation through universal run conditions compatible with all ResistancePlus® products.
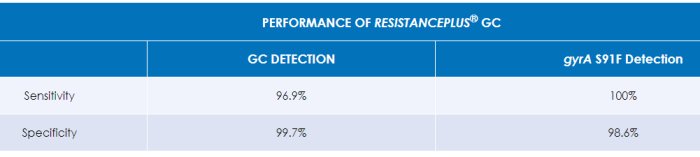
Demonstrated clinical performance – for more detail please see the ResistancePlus® GC Instructions for Use.
ResistancePlus® GC simultaneously detects the bacterium Neisseria gonorrhoeae (GC) and the gyrA S91 (wild type) or gyrA S91F (mutant) markers that are associated with susceptibility or resistance to the fluoroquinolone antibiotic, ciprofloxacin.
Single well: N. gonorrhoeae (Opa), N. gonorrhoeae (PorA), Ciprofloxacin resistance, Ciprofloxacin susceptibility, Internal control
Sample types
- Urine: Male and female
- Swabs: anal, rectal, cervical, endocervical, vaginal, urethral, pharyngeal, and eye
- Pre-extracted samples
Amplification instruments
- LightCycler® 480 Instrument II (LC480 II, Roche)
- Applied Biosystems® 7500 Fast (7500 Fast)
- Applied Biosystems® 7500 Fast Dx (7500 Fast Dx)
- Bio-Rad CFX96™ IVD (CFX96 IVD)
- Bio-Rad CFX96™ CFX96 Touch™ (CFX96 Touch)
Products are shipped on dry ice or ice gel packs.
Storage & stability
Expiry dates are stated on the labels. It is recommended that freeze/thaw cycles be limited to less than 15. Store protected from light at – 20°C.
Intended use
For in vitro diagnostic use. Not for sale in the USA.
Regulatory status
CE-IVD, TGA cleared
