

Cell Applications, Inc.
- Home
- Companies
- Cell Applications, Inc.
- Products
- Human Aortic Endothelial Cells: HAOEC
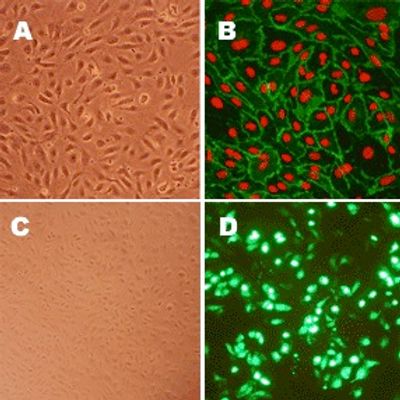
Human Aortic Endothelial Cells: HAOEC
Human Aortic Endothelial Cells (HAOEC) provide an excellent model system to study all aspects of cardiovascular function and disease, and they have been utilized in dozens of research publications to study diabetes-associated complications related to cardiovascular function, investigate mechanisms of immune response and graft rejection, study endothelial dysfunction caused by air pollution, oxidative stress and inflammation, and develop 3d endothelialized engineered tissues, as well as new technologies based on novel material surfaces and drugs in order to reduce risks associated with vascular implants. Select HAOEC lots have been additionally tested to demonstrate stimulation-dependent angiogenesis and key endothelial cell signaling pathways (phosphorylation of VEGFR, Akt, MAPK, and expression of Tie2, eNOS, Axl and Etk/Bmx). More information about pre-screened endothelial cells can be found on the Pre-Screened Endothelial Cell Product Page.
Most popular related searches
vascular implant
immune response
cardiovascular function
diabetic complications
diabetes complication
carotid artery
cardiovascular disease
veterinary use
coronary arterial
inflammation associated
- Demonstrate that increased glucose flux leads to endothelial dysfunction in diabetes via activating Egr1-mediated proinflammatory and prothrombotic responses
- Study apoptosis, oxidative stress and inflammation associated with atherosclerosis and demonstrate the beneficial effects of anthocyanin on endothelial cells damaged by exposure to oxidized sterols
- Demonstrate that upregulation of thioredoxin via AMPK-FOXO3 pathway protects endothelial cells from oxidative stress and may prevent cardiovascular diseases in patients with metabolic syndrome and diabetes and further elucidate the involvement of AMPK cascade in mediating beneficial cardiovascular effects of green tea
- Test anti-inflammatory and vasodilating properties of a synthetic rutaecarpine derivative
- Show that glycated albumin, associated with diabetic complications, decreases endothelial miR-146a expression which leads to increased IL-6 production, and that angiotensin protects endothelial cells by preventing miR-146a downregulation
- Demonstrate that air pollutants can directly affect ZO-1 function leading to increased endothelial permeability, inflammatory cell transmigration and initiation of atherosclerosis
- Discover the involvement of stress signaling JNK and p38 pathways in pathological suppression of thrombomodulin, a vascular protective molecule, downregulated in many thrombotic and vascular diseases
- Link uremic toxins (in particular, PAA) in patients with chronic liver disease to increased ROS production and stimulation of TNF-a in endothelial cells leading to atherosclerosis and vascular calcification
- Demonstrate that in diabetes, advanced glycation end products lead to ROS generation in endothelia via sustained NF-kB activation, contributing to progression of atherosclerosis
- Discover that CD40 ligand promotes monocyte adhesion to endothelial cells via PKCa, NF-kB and VCAM-1 signaling cascade, explaining the role of CD40L in atherogenesis
- Show that monocytes activated by endothelial cells, produce CD80 signaling that leads to allogenic immune response, indicating the need for specific therapy to prevent monocyte activation during allograft transplantation
- Identify tetraspanin CD82 as the recognition sensor responsible for rejection of xenotransplants
- Develop 3d endothelialized engineered tissues, as well as new technology based on novel material surfaces and drugs (such as paclitaxel, sirolimus, vitamin C, C6-ceramide and 17β-estradiol) to inhibit smooth muscle cell proliferation at the same time allowing endothelial cells adhesion and proliferation in order to reduce risk associated with vascular implants
Additionally, HAOEC (along with human subclavian artery (HScAEC), carotid artery (HCtAEC), coronary artery (HCAEC) and brachiocephalic artery (HBcAEC), all provided by Cell Applications, Inc.) have been used to demonstrate that not only blood vessels from different tissues are highly heterogeneous, they also interact differently with leukocytes during the inflammation response. The authors further showed that differential N-glycosylation of commonly expressed vascular adhesion molecules may be responsible for this heterogeneity, as well as for modulation of signaling under resting and activated inflammatory conditions. This also explains why specific vascular beds may be more or less susceptible to particular diseases or stimuli. Importantly, if cells from different sources were used, these results could not be convincingly validated due to a number of uncontrolled variables, such as age, race, genetic variability or life style choices of the donors. To eliminate the donor-to-donor variability, the scientists took advantage of the great variety of primary cells offered by Cell Applications, including the option of ordering a panel of endothelial cells obtained from different vascular beds of the same donor!
Because of the complex heterogeneity that exists not only between different donors, but even between different vascular beds in the same individual, it would be prudent to confirm any new findings on primary cell lots coming from several different origins.
Tissue: Normal healthy human aorta
QC: No bacteria, yeast, fungi, mycoplasma
Character: Factor VIII-related Ag, DiI-Ac-LDL uptake
Bioassay: Attach, spread, proliferate in Growth Med
Cryovial: 500,000 HAOEC (2nd passage) frozen in Basal Medium w/10% FBS, 10% DMSO
Kit: Cryovial frozen HAOEC (304-05a), Growth Medium (211-500), Subculture Rgnt Kit (090K)
Proliferating: Shipped in Tsfr Med, 3rd psg (flasks or plates)
Doublings: At least 16
Applications: Laboratory research use only (RUO). Not for human, clinical, diagnostic or veterinary use.
