


Calviri - Immune Checkpoint Inhibitors (ICI) Diagnostic
Calviri’s immunotherapy diagnostic is a huge leap forward in predicting therapy outcomes compared to current available solutions.
$25bn
ICI estimated market size
Immune Checkpoint Inhibitors are a leading cancer treatment with estimated total addressable market estimated at $25 billion per year – and growing
>50%
Cancer patients eligible for ICI
Over 50% of all cancer patients are already eligible for ICI treatment
2 (with more to follow)
Collaborations
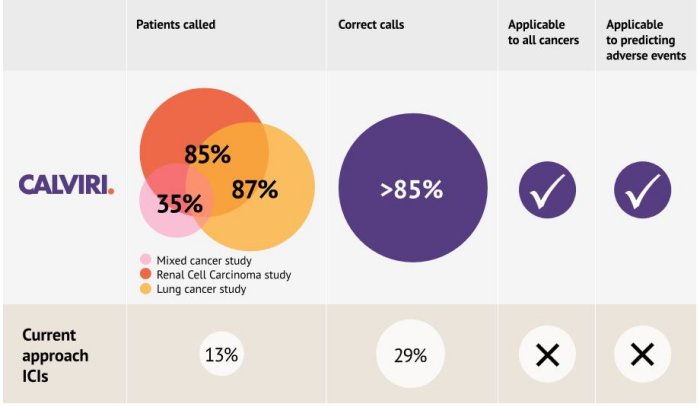
Calviri has established collaborations with two groups at MD Anderson, and are working on two others. Our preliminary results on renal cancer, and mixed rare cancers, using our Immunotherapy Response Predictor are positive – showing much improvement over the current standard
20-30%
Average response
Currently available ICI treatments have an average response of only 20-30%, and up to 70% adverse events
