- Home
- Companies
- TVAX Biomedical, Inc.
- Products
- TVI-BRAIN-1 - Immunotherapy for the ...
TVI-BRAIN-1 - Immunotherapy for the Treatment of Brain Cancer and Targets Glioblastomas
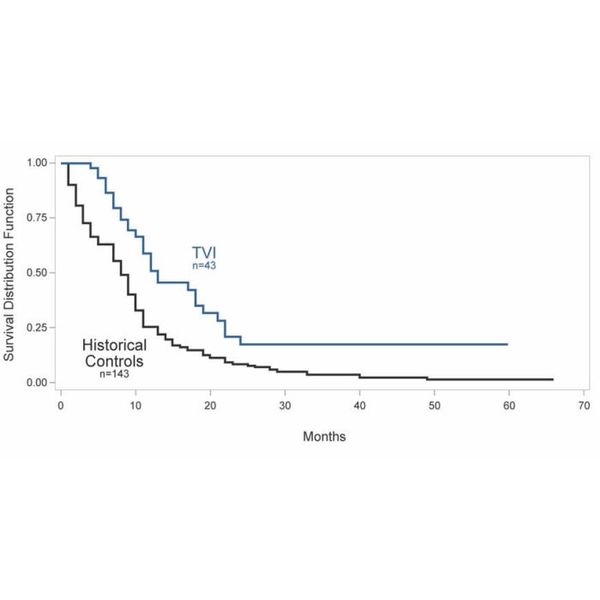
TVAX®’s lead candidate (TVI-Brain-1) is being evaluated for the treatment of brain cancer and targets glioblastomas. The drug candidate has received Fast Track Designation by the US Food and Drug Administration (FDA) to test TVI-Brain-1 in a Phase 2b clinical study for glioblastoma multiforme (GBM). The Fast Track Designation is supported by positive Phase 2 clinical data, as well as extensive preclinical and Phase 1 safety studies.
TVAX Immunotherapy has been evaluated in Phase 1/2 clinical trials in 43 patients with recurrent grade 3 and grade 4 gliomas who have previously failed surgery, radiotherapy and chemotherapy. Results of these studies have demonstrated significantly increased median survival for patients treated with TVAX Immunotherapy as compared to historical controls (see Kaplan-Meier survival curves below).