- Home
- Companies
- MitoImmune Therapeutics Inc.
- Products
- Model MIT-001 - IPF Pathology with ILD ...
Model MIT-001 - IPF Pathology with ILD Patients and PHMG Animal Model
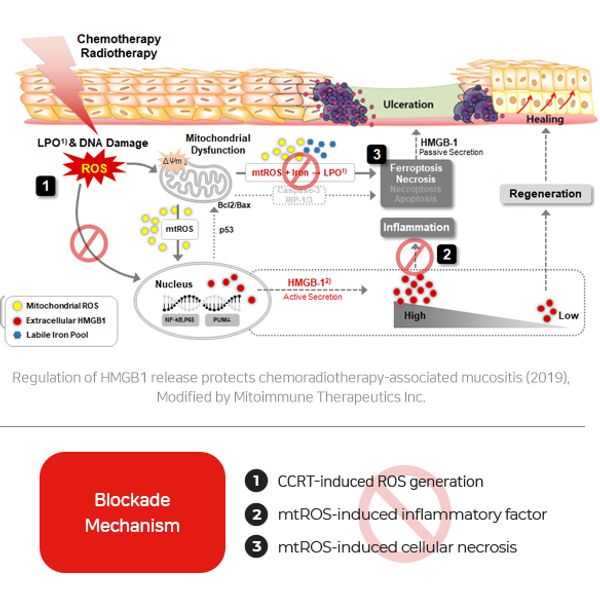
MIT-001 is expected to meet the medical need for oral mucositis for which there is no cure. It aims to be a first-in-class drug that can be used by both patients with head and neck cancer or hematological cancer with a high incidence and severity of oral mucositis as a side effect of chemoradiation therapy.
- The most common cause of pain during the treatment of cancer and the most serious side effect of chemo/ radiation therapy
- No approved treatment for oral mucositis in patients undergoing treatment for solid tumors
- Mucositis is inflammation and/or necrotizing ulcer of the mucous membrane of the digestive tract including oral cavity.
- → Oral mucositis increases the incidence risk of sepsis 4 times
- Due to the severe oral mucositis, discontinuation or delayed CCRT treatment, or decreased dose/intensity is chosen
MOA
Blocking the production of mtROS, the main cause of chemo/radio therapy-induced oral mucositis
Suppressing the production of DAMPs and cytokines, which are inflammatory factors, through inhibition of Ferroptosis & Necrosis
Efficacy
Applicable to treatment of oral mucositis in solid & hematologic cancer patients
Safety
Safety for long-term administration in cancer patients
Dosage
Applcationd to various dosing regimen
Intravenous or subcutaneous administration route
Strategy
- HNSCC and HSCT with very high incidence and severity of oral mucositis
- Fast track and breakthrough designation by US-FDA for accelerated IND and approval