Maxim Hiv Infected Equipment & Supplies
5 equipment items found
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
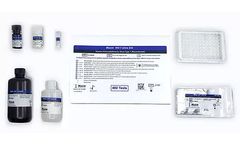
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim Swift HIV Recent Infection Assay (RIA) Kit Controls are quality control reagents for use only with the Maxim Swift™ HIV Recent Infection Assay (RIA) (P/N 92002). They are used to verify that the test kit reagents are working and that the test is performed correctly. The Maxim Swift™ HIV RIA ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
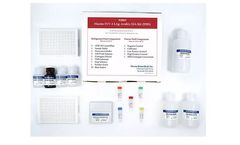
The Maxim HIV-1 Limiting Antigen-Avidity EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit HIV-1 specific IgG populations ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim HIV-1 Limiting Antigen-Avidity Dried Blood Spot (DBS) EIA is an in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term. Persons with recently acquired HIV-1 infections typically exhibit HIV-1 ...
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
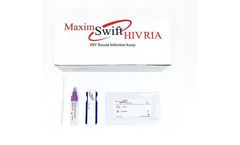
The Maxim Swift HIV Recent Infection Assay (RIA) is a single use qualitative immunoassay to detect the circulating antibodies to Human Immunodeficiency Virus Type 1 (HIV-1), Type 2 (HIV-2) and distinguish between recent and long-term infection in HIV-1/2. The assay is a point of care (POC) test ...