Refine by
Maxim Immunodeficient Equipment & Supplies
3 equipment items found
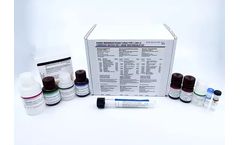
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
MaximBio's Cambridge Biotech HIV-1 Urine Western Blot Kit is an FDA approved in-vitro qualitative assay for the detection and identification of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) contained in human urine. This more specific assay is used as a supplemental test with urine specimens that tested repeatedly reactive using a screening procedure (e.g. FDA ...
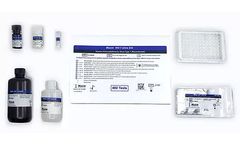
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
Maxim's FDA Approved HIV-1 Urine EIA test is an enzyme immunoassay for the in-vitro detection of antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) in urine. The test is intended for use in professional laboratory settings as an aid in clinical diagnosis of HIV infection. Before a determination of HIV-1 status can be made, specimens that are repeatedly reactive using this ...
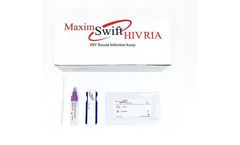
Manufactured by:Maxim Biomedical based inRockville, MARYLAND (USA)
The Maxim Swift HIV Recent Infection Assay (RIA) is a single use qualitative immunoassay to detect the circulating antibodies to Human Immunodeficiency Virus Type 1 (HIV-1), Type 2 (HIV-2) and distinguish between recent and long-term infection in HIV-1/2. The assay is a point of care (POC) test intended for use with blood or serum/ plasma specimens. The Maxim Swift™ HIV RIA ...