Show results for
Refine by
Qualitative Detection Of Antibodies Against Sars Cov 2 Equipment & Supplies In Cambodia
35 equipment items found
Premium
Manufactured by:Bertin Technologies based inMontigny-le-Bretonneux, FRANCE
BEC SARS-CoV-2 RT-LAMP system is designed for the rapid detection of SARS-CoV-2 genome on the field, in less than 30 ...
Manufactured by:GENCURIX based inGuro-gu, SOUTH KOREA
GenePro LAMP SARS-CoV-2 Test kit is a real-time reverse transcription loop mediated isothermal amplification (real-time RT-LAMP) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in upper respiratory specimens including oropharyngeal and nasopharyngeal swab, from individuals suspected of COVID-19 by their healthcare provider. It allows qualitative detection of RdRp gene ...
Manufactured by:Scandinavian Biopharma based inSolna, SWEDEN
SARS-CoV-2 Antigen Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasal swabs and nasopharyngeal swabs. The sensitivity is 92,00% and the specificity is 99.62%. The total duration of the test is 15-20 ...
Manufactured by:Vitrosens Biotechnology A.S. based inÜmraniye, TURKEY
RapidFor™ SARS-CoV-2 Rapid Antigen Test Kit enables in vitro qualitative detection of SARS-CoV-2 antigen, which results in COVID-19 infection. The RapidFor™ SARS-CoV-2 Rapid Antigen Test Kit uses the colloidal label as a tool for SARS-CoV-2 antigen detection in the collected nasopharyngeal, nasal, and oropharyngeal ...
Manufactured by:Sentinel CH. SpA based inMilano, ITALY
3-gene based Real Time RT-PCR for qualitative detection of SARS-CoV-2. STAT-NAT is Sentinel Diagnostics’ proprietary technology developed with a new protective compound able to stabilize the activity of enzymes for a very long time with no temperature controlled storage requirements. STAT-NAT kits contain all reagents and buffers required to perform the reaction. Lyophilized reagents are ...
Manufactured by:KH Medical based inPyeongtaek-si, SOUTH KOREA
RADI COVID-19 IgG/IgM Rapid test is a rapid immuno-chromatographic assay for qualitative detection of SARS-CoV-2-IgG & IgM antibodies in human serum, plasma or whole blood. RADI COVID-19 IgG/IgM Rapid test intend to use as an aid for identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior ...
Manufactured by:OPTOLANE Technologies Inc. based inSeongnam-si, SOUTH KOREA
The Genoplexor™ COVID-19 Detection Kit is an in vitro diagnostic kit designed for real-time reverse transcription polymerase chain reaction test intended for the qualitative detection of SARS-CoV-2 (RdRP gene, S gene) RNA in human samples such as human upper respiratory (oropharyngeal swab and nasopharyngeal swab) from individuals with suspected or confirmed respiratory ...
Manufactured by:Tersaco AG based inImmensee (SZ), SWITZERLAND
SARS CoV-2 IgG/IgM Rapid Test is a chromatographic lateral flow immunoassay for the qualitative detection of anti-SARS-CoV-2 IgG and IgM in human whole blood, serum or plasma ...
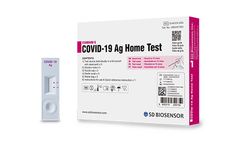
Manufactured by:SD Biosensor based inSuwon-si, SOUTH KOREA
STANDARD Q COVID-19 Ag Home Test is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 nucleocapsid antigen present in human nasal ...
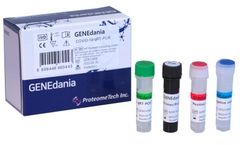
Manufactured by:PROTIA. iNC, formely known as ProteomeTech Inc. based inSeoul, SOUTH KOREA
GENEdania COVID-19 qRT-PCR is an in vitro diagnostic kit for the qualitative detection of COVID-19 (SARS-CoV-2) RNA in human upper and lower respiratory specimens using real-time reverse transcription polymerase chain reaction (qRT-PCR) ...
Manufactured by:HymonBio Co.,Ltd based inSuzhou, CHINA
Hymon COVID-19 antigen rapid test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) with nasal swabs or saliva during the acute phase of infection. It only takes 15 minutes to complete the test and the test is also non-invasive. Negative results do not rule out SARS CoV-2 infection, and should not be used as the sole basis for treatment. ...
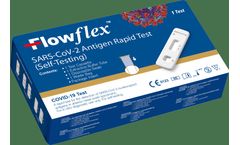
Manufactured by:AusDiagnostics based inMascot, AUSTRALIA
The Flowflex™ SARS-CoV-2 Antigen Rapid Test (Self-testing) allows qualitative detection of the SARS-CoV-2 nucleocapsid antigen in just 15 minutes. Designed to be easy-to-use and rigorously tested, the Flowflex™ self-test kit is trusted by major clinics around the world to provide accurate detection of SARS-CoV-2. ...
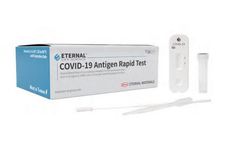
Manufactured by:Zinexts Life Science Corp. based inNew Taipei City, TAIWAN
The Eternal COVID-19 Rapid Antigen Test is a lateral flow immunoassay intended for the qualitative detection of SARS-CoV-2 antigens in the nasopharyngeal and nasal swab samples. The Eternal COVID-19 Rapid Antigen Test has a sensitivity and specificity comparable to PCR tests (98%~99%; 194 positive & negative samples.), and higher detection sensitivity compared to industry-leading ...
Manufactured by:Convergent Technologies GmbH & Co. KG based inCoelbe, GERMANY
Convergys® POC RT-PCR COVID-19 Detection Kit.m for use with the Convergys® POC RT-PCR Nucleic Acid Detection System. The COVID-19 respiratory disease is caused by infection with the novel coronavirus SARS-CoV-2. The symptoms of COVID-19 are quite unspecific and resemble e.g. those of Influenza. Furthermore, in many cases the manifestation of symptoms is very weak. Hence, proper ...
Manufactured by:RapiGEN Inc. based inSuwon-si, SOUTH KOREA
One Step SARS-CoV-2 Antigen Rapid Test. BIOCREDIT COVID-19 Ag Test Nasal is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 antigen in human nasal specimen. This test is for in-vitro professional diagnostic use and intended as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms. It provides only an initial screening test result and ...
Manufactured by:Molbio Diagnostics Pvt. Ltd. based inVerna, INDIA
Intended Use: A lyophilized, ready-to-use, open format, duplex Real Time Reverse Transcription Polymerase Chain Reaction (RT PCR) test for the qualitative detection of SARS CoV-2 RNA in human oropharyngeal and nasopharyngeal swabs and aids in the diagnosis of COVID-19. The test detects the E and Orf1a genes of the virus. Truemix COVID-19 runs on standard Real Time PCR ...
Manufactured by:Gold Standard Diagnostics based inDavis, CALIFORNIA (USA)
The GSD NovaType Detect + Select K417N SARS-CoV-2 is a research use only (RUO) real-time reverse transcription PCR (RT-PCR) assay designed for the simultaneous qualitative detection of SARS-CoV-2 (Severe acute respiratory syndrome coronavirus 2) genomic RNA and identification of the spike (S) gene mutation K417N. It is intended to aid in the identification of SARS-CoV-2 variant of concern (VOC) ...
Manufactured by:RapiGEN Inc. based inSuwon-si, SOUTH KOREA
One Step SARS-CoV-2 Antigen Rapid Test. BIOCREDIT COVID-19 Ag Home Test Nasal is a rapid chromatographic immunoassay intended for the qualitative detection of SARS-CoV-2 in individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection. This test is authorized for home use with self-collected direct nasal (nares) samples using the sterilized nasal swab ...
Manufactured by:NOWDiagnostics Inc. based inSpringdale, ARKANSAS (USA)
The ADEXUSD COVID-19 Test is an in vitro lateral-flow immunoassay intended for qualitative detection of total antibodies to SARS-CoV-2 in human venous whole blood (dipotassium EDTA) and plasma (dipotassium EDTA), serum, and fingerstick whole ...
Manufactured by:Amoy Diagnostics Co., Ltd. based inXiamen, CHINA
The novel coronaviruses belong to the p genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. ...