- Home
- Equipment
- canada northwest territories
- vascular testing system
Refine by
Vascular Testing System Equipment & Supplies In Canada Northwest Territories
58 equipment items found
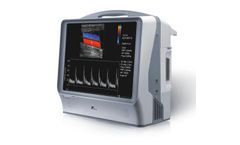
Manufactured by:Canadian Hospital Specialties Limited (CHS) based inOakville, ONTARIO (CANADA)
The Smartdop® XT6 is the ideal vascular ABI and PPG toe pressure Doppler. With 6 inflation ports, a simple one-button start, and dual side cuff inflation, the Smartdop® XT6 can perform a complete bilateral ABI study in minutes. Integrated pulse volume and PPG (photoplethysmography) probes enable a wide variety of arterial and venous studies for accurate assessment of vascular disease. ...
Manufactured by:RTI Group AB based inMölndal, SWEDEN
The GE Vascular Holder is designed for accurate positioning of the Piranha and Barracuda Dose Probes on the GE Vascular ...
Manufactured by:Osypka AG based inRheinfelden, GERMANY
OSYPKA Snare Catheter is a snare catheter with a loop that can be extended smoothly up to 40 mm in diameter. It is made out of highly flexible, buckle-resistant and shape-maintaining ...
Manufactured by:Universal Diagnostic Solutions based inVista, CALIFORNIA (USA)
The Vista ABI system was designed for practices that want to begin performing ABI exams now and also have the option of later addressing accessories like the PPG probe and software. The accompanying compact printer rapidly prints two ankle waveforms along with the ABI ...
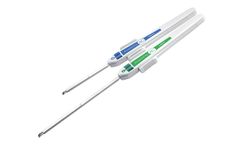
Manufactured by:Remington Medical Inc. based inAlpharetta, GEORGIA (US) (USA)
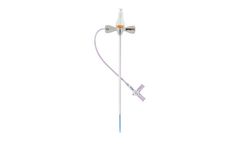
Introducer set used for placement of a .038-inch diameter guide wire into the vascular ...
Manufactured by:PFM Medical, Inc. based inCarlsbad, CALIFORNIA (USA)
The Multi-Snare Device is a patented, highly efficient multiple loop retrieval snare with a dual-plane design to grasp objects from numerous angles. Designed with various reliable pull-force strengths, the kink resistant nitinol dual loop system grants the ability to grasp, manipulate or assist from any angle or vessel sidewall. The Multi-Snare MAX Device has been designed with the same ...
Manufactured by:Shenzhen Delica Medical Equipement Co.,Ltd based inGuangming District, CHINA
Integrated solution: Robotic probe monitoring system-advanced robotic probe system, with function of automatic scan and track the blood flow signal and make the application of TCD long term monitoring become realistic. Bedside Diagnosis-Equipped with hign frequency linear array probe, low frequency convex array probe and phased array probe, patients can be examined by bedside ultrasound and ...
Manufactured by:Integer Holdings Corporation based inPlano, TENNESSEE (USA)
Introducing the Adelante® SafeSheath® II Hemostatic Peel Away Introducer System for Vascular Access. This generation peel away introducer features the latest lubricated hemostatic valve membrane that provides low insertion forces during procedures. It also includes a side port with a 3-way stopcock that provides a convenient means of aspirating and flushing the ...
Manufactured by:Cordis based inMiami Lakes, FLORIDA (USA)
Intended for use in the intravascular introduction of interventional or diagnostic devices for coronary or peripheral vascular ...
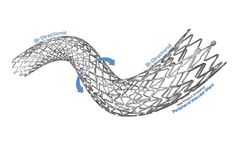
Manufactured by:Stron Medical, Part of Q3 Medical Group based inWinsen, GERMANY
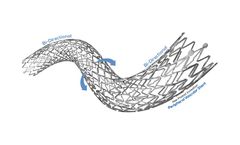
The POLARISADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...
Manufactured by:THERA-Trainer UK Ltd. based inMilton Keynes, UNITED KINGDOM
The THERA-Trainer coro is a therapy device for safe dynamic exercise of balance (postural control). It is suitable for patients able to stand and was designed for the daily use in clinics and therapy facilities as well as for use at home. It is in accordance to the most recent quality and safety ...
Manufactured by:Universal Diagnostic Solutions based inVista, CALIFORNIA (USA)
This full-featured vascular assessment system is ideally suited to perform segmental exams. The Vista AVS offers three modalities for obtaining systolic pressures and waveforms and is designed to accommodate both standard and custom ...
Manufactured by:Tsunamed, Part of Q3 Medical Group based inWinsen, GERMANY
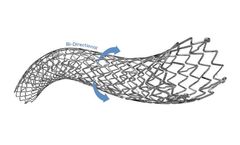
The NAVALIS-ppADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...
Manufactured by:Stron Medical, Part of Q3 Medical Group based inWinsen, GERMANY
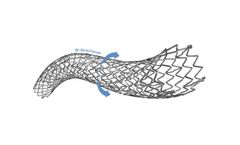
The POLARIS-ppADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...
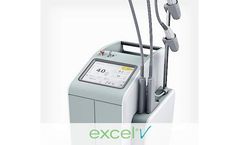
Manufactured by:Cutera, Inc. based inBrisbane, CALIFORNIA (USA)
The first laser to feature 2 ultra-precise, best-in-class wavelengths (532/1064 nm) and 3 unique operating modes to treat a wide range of superficial and deep vascular lesions, pigmentary issues and skin revitalization concerns safely and effectively. Applications include rosacea, to diffuse redness, facial veins, leg veins, periorbital veins, angiomas, port wine stains, poikiloderma, wrinkles, ...
Manufactured by:SyncMedical LLC based inStoughton, MASSACHUSETTS (USA)
The Primo Port is a fully implantable vascular access device that meets the requirements of clinicians and patients by providing long-term repeated access to the vascular system. The Primo Port system can be used for infusion of medications, IV fluids, parenteral nutrition and blood products as well as for the aspiration of blood ...
Manufactured by:Tsunamed, Part of Q3 Medical Group based inWinsen, GERMANY
The NAVALISADVANCE Peripheral Vascular Self-Expanding Stent System combines highly durable nitinol stent with a flexible delivery system to provide proven clinical ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
The AnastoClip AC Closure System provides precise and rapid vascular anastomosis. AnastoClips, placed in an interrupted fashion, facilitate a compliant anastomosis without penetrating the vessel lumen. The anastomosis is allowed to contract and expand to accommodate pulsative flow due to interrupted AnastoClip placement. In addition, hemostatic anastomosis and faster clipping reduce OR time. The ...
Manufactured by:Fisioline S.r.l. based inVerduno, ITALY
LINFOPRESS MASTER is the latest development in peristaltic differentiated-sector pressure therapy. This type of device allows acting physiologically wherever helping for problems due to an incorrect functioning of the lymphatic or venous system is needed. LINFOPRESS MASTER exploits a powerful and silent compressor in order to obtain inflating cycles with an accurate pressure regulation ...
Manufactured by:LeMaitre Vascular, Inc. based inBurlington, MASSACHUSETTS (USA)
The AnastoClip GC Closure System is designed to create a more secure and compliant vascular anastomosis. The anastomosis is allowed to contract and expand to accommodate pulsatile flow due to interrupted AnastoClip GC Clip placement. The AnastoClip GC Closure System now can also be used for approximation of dural tissue. The new 15cm length allows for better access in hard to reach anatomies. ...