

- Home
- Companies
- EntroGen, Inc.
- Products
- EntroGen - Model KRAS-RT50 - KRAS ...
EntroGen - Model KRAS-RT50 - KRAS Mutation Analysis Kit for Real-Time PCR (exons 2, 3 and 4)
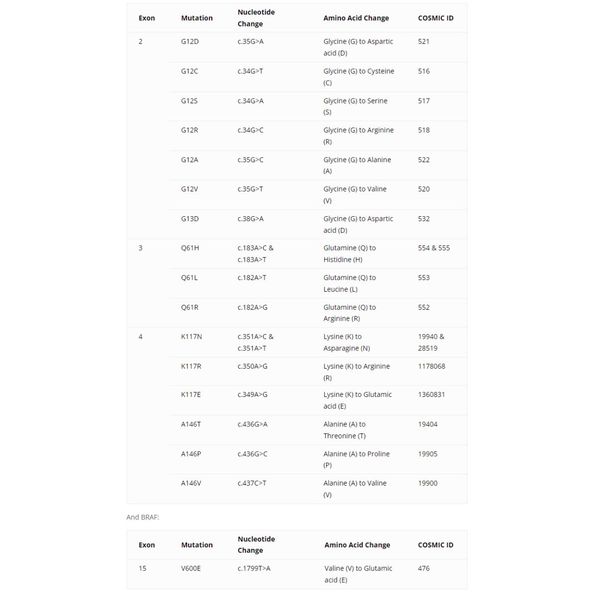
The KRAS gene encodes a small GTPase that plays a key role in transducing signals from the epidermal growth factor receptor (EGFR) to downstream effectors. KRAS mutations have been commonly found in several types of human malignancies, such as metastatic colorectal cancer (mCRC), lung adenocarcinoma and thyroid cancer. The most common mutations are found in codons 12, 13 and 61. Several studies have demonstrated that tumors carrying any of these mutant forms of the KRAS gene are less likely to respond to anti-EGFR antibody therapy.
The American Society of Clinical Oncology (ASCO) recently released its first Provisional Clinical Opinion (PCO) suggesting that all patients to be administered anti-EGFR monoclonal antibody therapy (e.g. cetuximab, panitumumab and erlotnib) should be screened for KRAS mutations. Recent studies have also shown that not all mCRC patients with wild-type KRAS tumors respond to anti-EGFR therapy. This suggests that additional genes and/or pathways may be involved in the mechanism of resistance to these drugs. Mutations in BRAF, another downstream effector of the EGF-activated pathway, have been identified in up to 8% of mCRC tumors. Studies with mCRC patients have shown resistance to anti-EGFR therapy in patients with tumors expressing mutated BRAF. Those same individuals also had decreased progression-free (PFS) and overall (OS) survival when treated with EGFR antagonists. These findings strongly suggest that screening for both KRAS and BRAF mutations is necessary to more accurately identify tumor cells that will not respond to anti-EGFR drugs.
- Limit of Detection at less than 1%
- Highly sensitive and specific
- Works with FFPE, fresh frozen and FNA samples
- Simple setup and interpretation
- Works on most real-time PCR instruments
- Available as RUO and CE-IVD
For expanded coverage, the RAS c.59/117 Mutation Detection Kit detects KRAS c.59, NRAS c.59/117 somatic mutations.
Testing Procedure and Analysis
EntroGen’s KRAS/BRAF mutation panel is a polymerase chain reaction (PCR)-based assay and uses allele-specific probes to identify the presence of mutations in KRAS exons 2, 3 and 4 as well as BRAF V600E. The testing procedure involves four (4) simple steps:
- Isolation of DNA from tumor biopsies, paraffin-embedded sections (FFPE), fresh frozen tumors, or tumor cell lines.
- Amplification of regions of the KRAS and BRAF genes using allele-specific probes.
- Detection of amplification signal using real-time PCR instrument or gel documentation system.
- Documentation and interpretation of results.
This test can be completed in approximately 2 hours from DNA to test result.
Equipment and Materials
This assay requires a real-time thermal cycler capable of detecting FAM and VIC fluorescence signals. This test includes reagents required for the PCR amplification and signal detection, as well as validated reaction controls. Columns and reagents for DNA isolation are not included.
Intended Use
USA: EntroGen’s KRAS/BRAF mutation panel reagents are provided for research use only (RUO). Not for use in diagnostic procedures.
Europe: EntroGen’s KRAS/BRAF mutation panel reagents are available for research (RUO) and diagnostic (CE-IVD) purposes.
