

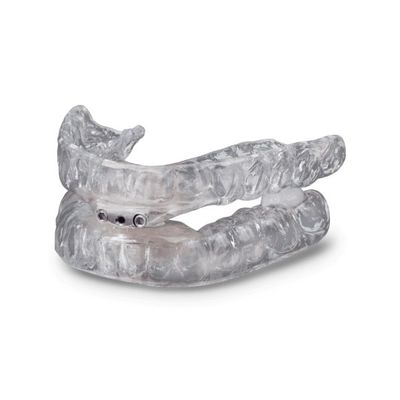
Glidewell - Model TAP 3 TL - Mandibular Advancement Devices
Helping patients who suffer from sleep-disordered breathing, the TAP 3 TL is part of the TAP family of appliances for the treatment of snoring and obstructive sleep apnea (OSA). Based on the same principle as cardiopulmonary resuscitation (CPR), the TAP (Thornton Adjustable Positioner) was designed to keep the airway open to allow for air to pass. A constricted or collapsed airway causes snoring and OSA. The TAP device holds the lower jaw in a forward position, maintaining a clear airway to reduce snoring and improve breathing. Fitting comfortably, the TAP 3 TL is designed to provide room for the tongue and allow the lips to close. An adjustment key enables the patient to adjust the protrusion of the lower jaw while wearing the device until a comfortable and effective position is achieved.
Oral appliance therapy (OAT) is very effective in treating patients with sleep-disordered breathing, with a compliance rate shown to be as high as 90% over a 2.5-year period.
OAT is associated with greater patient satisfaction than nasal continuous positive airway pressure (CPAP) therapy.
In a randomized crossover trial comparing OAT to CPAP in patients with obstructive sleep apnea, about 81% preferred OAT.
Among patients with obstructive sleep apnea, both continuous positive airway pressure (CPAP) and mandibular advancement devices (MADs) were associated with reductions in blood pressure. Network meta-analysis did not identify a statistically significant difference between the blood pressure outcomes associated with these therapies.
A mandibular advancement device for obstructive sleep apnea reduces nocturnal blood pressure in women.
- Indications : The TAP 3 TL is intended to reduce or alleviate nighttime snoring and sleep-related breathing disorders, including obstructive sleep apnea (OSA).
- Material Composition : Thermoformed triple laminate and cobalt-chrome alloy hardware
- In-Lab Working Times : 5 days
- Available Colors : Clear
- Policies & Warranty :
- NO-FAULT REMAKE POLICY: Glidewell is pleased to process all remakes or adjustments at no additional charge if requested within the warranty period and accompanied by the return of the original appliance.
- LIMITED WARRANTY/LIMITATION OF LIABILITY. Glidewell (“the lab”) warrants that all dental devices (a “device”) are made according to your specification and approval in the belief that the device will be useful and MAKES NO OTHER WARRANTIES INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Subject to the return of a device that is placed and then fails, the lab will repair or replace the device without charge for the cost of materials and workmanship or refund the original price paid, at the lab’s option, for up to 2 years for the TAP 3 TL appliance.
