

- Home
- Companies
- Vitalis, LLC
- Products
- Vitalis - Model VTS-72 - Novel Fumarate ...
Vitalis - Model VTS-72 - Novel Fumarate + VTS-Aspirin for Relapsing-Remitting Multiple Sclerosis (RRMS)
VTS-72 aims to be the next generation oral fumarate therapy, that may hold potential as a treatment for RRMS with fewer side effects and other clinical benefits for these patients.
Multiple Sclerosis (MS) is a debilitating chronic disease that affects millions of patients worldwide (Feigin et al.), in which the body’s own immune system attacks the central nervous system, causing irreversible damage that eventually leads to a slow but steady decline in mobility and life.
In the last 20 years, MS treatment has been revolutionized, first with injectables and more recently with oral medications, including fumarates. Dimethyl fumarate (DMF) is currently the leading oral treatment for MS.
However, its most common side effect (Bomprezzi 2015), flush, can cause discomfort and distress among patients and result in discontinuation and under-dosing (Sejbaek et al.). Aspirin has been used as a pre-treatment to reduce the Fumarate Flush. However, patients would have to take it at least 30 minutes before each administration and compliance has been poor.
A pilot study suggests lower flush
In addition, 39% of subjects who had experience flush with DMF reported absolutely no flush after VTS-72.
In a randomized, open-label, 2-way crossover study of 18 healthy subjects using the Global Flush Severity Scale (GFSS), subjects experienced a statistically significant average 63.3% less flush after VTS-72 than after standard dimethyl fumarate (DMF) (p=0.0018).
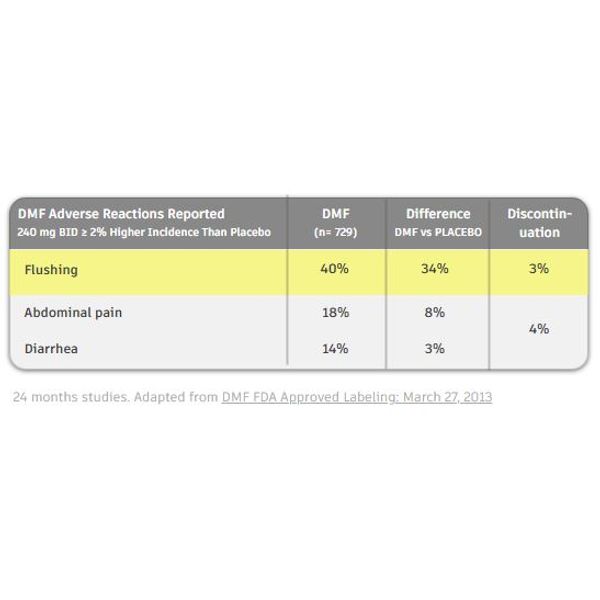
Post-marketing studies have shown the following:
- Mallucci et al.
- 27-29% discontinuation at 12 months.
- Duquette et al.
- 44% discontinuation at 24 months.
- Eriksson et al.
- 57% discontinuation at 24 months.
