

Lifetech Scientific (Shenzhen) Co., Ltd.
- Home
- Companies
- Lifetech Scientific (Shenzhen) Co., ...
- Products
- Cera - Model PDA - Occluder

Cera - Model PDA -Occluder
Nitinol wire frame coated with Titanium Nitride (TiN). Decrease the dissolution of nickel ion efficiently, expected safe long-term biocompatibility. Promote the growth of endothelial tissue, lessen thrombus complication. Superior superelastic, effectively reduce atrioventricular block occurrence.
Most popular related searches
Strategic Membrane Selection
- The ASD/PFO covered by a PET membrane that minimizes the chance of clot formation and has a small volume to get into lower profile sheath.
- The VSD/PDA occluder covered with a PTFE membrane, which has a denser structure suited for high pressure defect.
Conform anatomical features of the defect, providing optimal design
- 3 types of peri-membranous VSD and a muscular VSD devices designed for different kind of VSD.
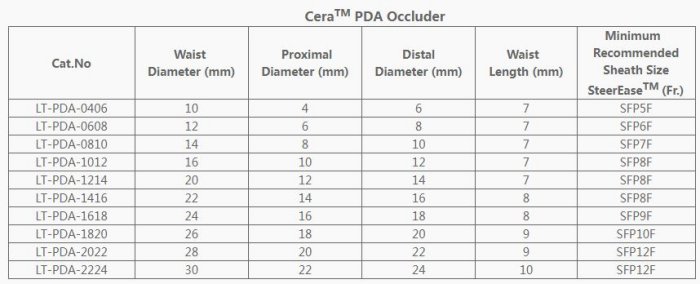
- The ASD waist diameter available ranging from 6 to 42 mm, and the PDA occluder ranging from 0406 to 2224.
Cera™ Clinical study in China:
- Principal Investigator: Zhang Zhiwei, MD, FACC, Guangdong Cardiac Institute.
- Evaluation of efficacy and safety of Cera™ septal defect occluder for congenital cardiac detect: A multicenter, randomized and controlled clinical trial.
- 11 medical centers,460 cases enrolled (231 Cera™, 229 HeartR™).
- Follow up endpoint: 1, 3, 6, 12 months.
Study conclusion:
- The success rate of immediate complete occlusion with Cera™ occluder is higher than 97.8%.
- The results showed that the incidence of residual shunt were relatively less.
- The arrhythmia incidence in Cera group is lower than that in HeartR group which as much as 36%.
Cera™ PM-VSD clinical study in Brazil:
- Principal Investigator: Doctor Raul Arrieta and tutored by Doctor Carlos Pedra.
- Prospective, multicenter, non randomized study.
- Starting on November 2010.
- 3 Brazilian institutions, 56 patients.
- Follow up endpoint: 1, 3, 6, 12 and 24 months.
Initial Result:
- The percutaneous closure of peri-membranous ventricular septal defect with Cera™ Device has showed a safe effect with excellent immediate occlusion and low complication.
