

Cobalt Light Systems
- Home
- Companies
- Cobalt Light Systems
- Products
- Cobalt - Model TRS100 - Pharmaceutical ...

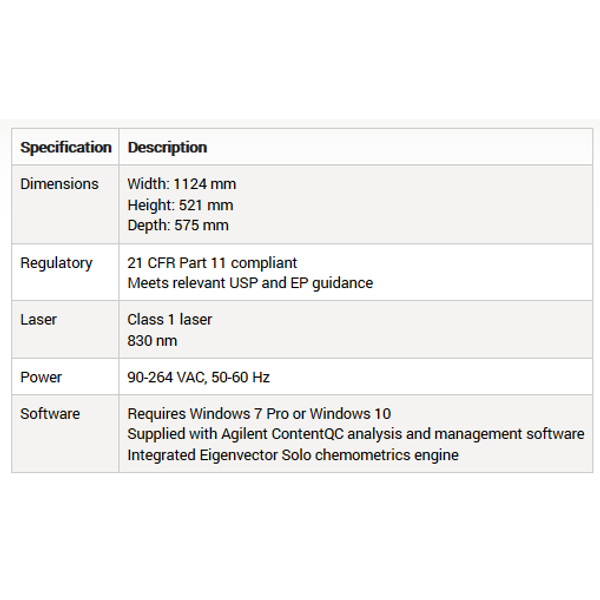
Cobalt - Model TRS100 - Pharmaceutical Quality Control System
The TRS100 enables fast, easy to use whole tablet or capsule assay & polymorph analysis for pharmaceutical QC testing. Easier to implement than other spectroscopic methods, Agilent`s transmission Raman spectroscopy (TRS) technology allows simple method-development and deployment in QC applications. A TRS100 system can reduce content uniformity (CU), assay, and ID tests to minutes per batch, saving significant cost and speeding up your QC workflow.
Most popular related searches
active pharmaceutical ingredient
pharmaceutical analysis
pharmaceutical ingredient
pharmaceutical quality
pharmaceutical testing
Content Uniformity Testing
- Whole tablet/capsule analysis – no sub-sampling
- Works with thick intact samples
- No sample preparation, wet chemistry or skilled testing resource
- LOQ 0.1-1% w/w
- Methods approved by regulators
Transmission Raman spectroscopy (TRS) is ideal for high throughput content uniformity analysis. A TRS measurement takes only a few seconds per sample with good accuracy, precision and linearity of response. TRS technology is specific and sensitive for nearly all APIs, excipients and lubricants with low sensitivity to water/moisture, particle density or surface coatings. Spectral features are sharp, distinct and easy-to-interpret and assign to each formulation component.
Polymorph Quantification
Most active pharmaceutical ingredients (APIs) are crystalline materials with a well-defined polymorphic form. Manufacturing or processing can change that form, and increasingly, amorphous APIs are used by formulators to increase bioavailability. The TRS100 can directly measure low-energy crystalline vibrational modes (the phonon mode region) to quantify polymorph content in seconds and to a high precision in intact, formulated samples.
The most common technique for quantitative polymorph analysis is XRPD, which has a sensitivity limit of around 5% w/w and requires tablets to be crushed and sampled. The TRS100 can detect residual polymorphs in a fully formulated amorphous API tablet with a limit of detection (LOD) of 0.1-1% w/w. The LOD is comparable with ssNMR but requires no sample preparation, is much quicker and requires less resource.
- Assay hundreds of tablets or capsules in minutes
- Works with intact capsules and coated tablets of most thicknesses
- No sample preparation, wet chemistry or skilled testing resources needed
- Quantifies APIs and excipients in a single measurement
- Limit-of-Quantification as low as 0.1% w/w
- Quantifies polymorphs in intact tablets
- Lean calibration models - easy to build and maintain
- Methods approved by regulators
- A range of sample trays and accessories for tablets, capsules, liquids and powders are available
