Cellphire Therapeutics, Inc.
- Home
- Companies
- Cellphire Therapeutics, Inc.
- Products
- Cellphire - Pipeline of Cellular ...
Cellphire - Pipeline of Cellular Therapies
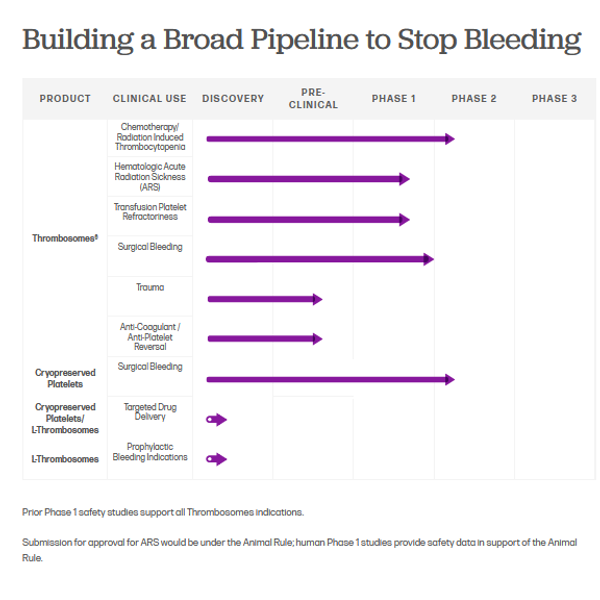
Cellphire Therapeutics is committed to transforming hemostasis management with innovative products that fill critical gaps in the treatment of bleeding patients. Our lead clinical-stage candidate, Thrombosomes, is an activated platelet-based hemostatic. The product is currently in a Phase 2 clinical trial in bleeding thrombocytopenic patients. Thrombosomes has also been given orphan drug designation for the treatment of Acute Radiation Sickness by the U.S. Food and Drug Administration.
Most popular related searches
platelet based
drug designation
drug administration
clinical stage
hemostasis management
regenerative medicine
drug delivery
clinical trial
cell therapy
platelet
Cellphire is identifying and developing other novel platelet-based therapies to address unmet needs. We’re researching transformative roles for platelets in targeted drug delivery, anti-thrombotic reversal, and regenerative medicine, among others.