- Home
- Companies
- ProUroCare Medical Inc.
- Products
- ProUroScan - Prostate Imaging System

ProUroScan - Prostate Imaging System
ProUroScan is an advanced medical imaging system that uses an array of sensors mounted on a rectal probe, a central processing unit and software and image construction algorithms to provide a real time color image of abnormalities in the prostate.
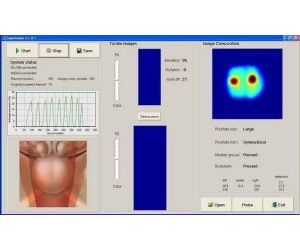
The system provides an image or record of the pressures that are generated from palpation of the posterior surface of the prostate using a rectal probe. The system’s operation is based on measurement of the stress pattern created when the probe is pressed against the prostate through the rectal wall. Temporal and spatial changes in the stress pattern provide information on the elastic structure of the gland and allow two-dimensional reconstruction of prostate anatomy and visualization of prostate mechanical properties. The prostate image is displayed on a screen that allows physicians to visualize tissue abnormalities in the prostate gland. In addition to the real time visual image, the results are stored electronically as a digital record.
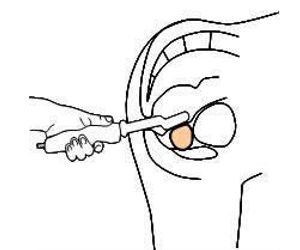
The ProUroScan System probe is specially designed for the rectal anatomy to minimize patient discomfort. It is ergonomic for the clinician and similar to a traditional DRE for the patient. The probe utilizes highly sensitive pressure sensors located on the face of the probe head to palpate the prostate. The probe’s positioning system ensures that the person administering the scan examines the entire surface of the prostate, and assists prostate image construction.
To perform a scan, the clinician inserts the tip of the probe into the patient’s rectum and palpates the prostate. As the prostate is palpated, an image of the prostate is produced and displayed on the computer monitor, along with indicators of the amount of pressure being applied to help guide the clinician. The image that is generated during the evaluation shows the physician in real-time where abnormal tissue exists in an otherwise homogeneous soft tissue organ.
Mechanical or elasticity imaging refers to a non-invasive analysis of tissue movement and displacement. The ProUroScan technique works by computing how tissue moves in response to pressure, thus evaluating its softness or stiffness. Sensors on the head of the probe collect a sequence of pressure patterns while the probe is pressed against the prostate. The device consequentially measures the prostate’s elasticity. Each scan produces an image of the prostate and compares elasticity measurements across the gland.
The image of the prostate that is created is designed to identify variations in tissue elasticity using a specially designed rectal probe. Once the image is created it can be utilized by the physician to assist in evaluating the results of an abnormal digital rectal exam for men and stored as an electronic record. Tissue that is confirmed by DRE as abnormal will exhibit less elastic properties and be represented by progressively darker areas on the image of the map as compared to normal tissue. During the real-time imaging examination, the physician can direct the probe to specific areas of interest as confirmed by DRE. The final composite image is saved in a file as a permanent electronic record and can be conveniently retrieved to view previous test results.
Prostate cancer is the most common form of cancer and the second leading cause of cancer death in men over the age of 50. The National Cancer Institute estimates that approximately 241,000 men were diagnosed with prostate cancer and over 33,000 died from the disease in 2011.
Screening. Currently, there are approximately 42 million men in the U.S. over the age of 50. For men in this age category, the standard of care to screen for the presence of prostate cancer is to have a physical exam each year in which two tests are routinely performed, the digital rectal examination for men (“DRE”) and the Prostate Specific Antigen (“PSA”) blood test. Although used for many years, the specificity of these tests has been widely questioned. Data from community based studies suggest that the positive predictive value of a DRE for prostate cancer is 15% to 30% and varies relatively little with age. For elevated PSA levels between 4 and 10ng/mL, the positive predictive value is approximately 20%. For studies in which biopsies were done when the results of either test were abnormal, 18% to 26% of screened patients had suspicious results, cancer was actually detected in approximately 4% of screened patients and the positive predictive value of the tests combined was 15% to 21%. In another study involving 6,630 volunteers, the combination of DRE and PSA detected 26% more cancers than PSA alone. Although PSA and DRE provide some positive predictive value, neither of these tests creates a physical or visual record documenting abnormalities in the prostate.
Prostate Cancer Detection. If a patient is suspected of having prostate disease as a result of standard screening tests, he is generally referred to a urologist. A urologist will usually perform his own digital rectal exam for men and may decide to perform a prostate biopsy to obtain tissue samples for microscopic analysis. The prostate biopsy is conducted by a needle that is guided by ultrasound into the prostate through the rectal wall. Since the existence and exact location of possible cancerous tissue is not known, the urologist will usually take 10 to 14 samples in a scattered pattern throughout the prostate in an attempt to find the suspect tissue. Of the approximately 1 million prostate biopsy procedures done each year in the United States only approximately 25 percent actually detect the presence of cancer. The low predictive ability of the DRE and PSA tests to gauge the presence of cancer over-inflates the number of referrals for invasive biopsy than are necessary to confirm that a patient has cancer.
Prostate Cancer Treatment. The treatment path for patients who test positive for prostate cancer depends on many variables, including age, location and pathology of the cancerous tissue and general health of the patient. Generally, a younger, otherwise healthy patient will elect to have a radical prostatectomy to eliminate the possibility that it might spread beyond the prostate. Older, less healthy patients may elect not to undergo surgery, and instead monitor the disease closely by semi-annual PSA and DRE exams, and annual biopsies. This monitoring regimen is commonly referred to as “active surveillance.” Some patients may elect radiation or drug treatments, in addition to necessary ongoing active surveillance. The National Cancer Institute estimates that there are approximately 2.4 million men alive who have a history of cancer of the prostate.

Tissue Displacement
Tissue displacement occurs anytime body tissue moves in response to pressure. As areas of abnormal tissue form within an organ, the tissue often becomes more dense and less elastic. When pressure is applied to the organ, abnormal tissue generally exhibits less displacement than normal tissue. This difference can be detected and measured.
Mechanical imaging is a non-invasive analysis of tissue movement and displacement. The ProUroScan technique works by computing how tissue moves in response to pressure, this evaluating its softness or stiffness. During a procedure similar in nature to the digital rectal examination, the clinician inserts the tip of the system’s probe into the patient’s rectum and palpates the prostate. Sensitive sensors on the head of the probe collect a sequence of pressure patterns when the probe is pressed against the prostate. The device consequently measures the prostate’s elasticity. Each can produces an image of the prostate and compares elasticity measurement across the gland.
The technology employed is based on work originally performed by Artann Laboratories Inc. (Trenton, NJ) under a series of government grants, including a $3 million Small Business Innovation Research Phase II Competitive Renewal grant received in 2006 from the National Institute of Health and the National Cancer Institute. This grant was made specifically to advance the development and application for clearance of the ProUroScan System by the FDA. We have entered into license and development and commercialization agreements with Artann relating to their existing technology and know-how and all future technology developed by Artann in our field of use.
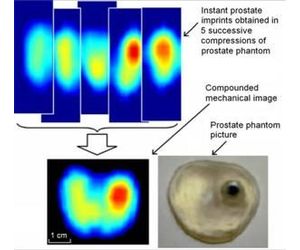
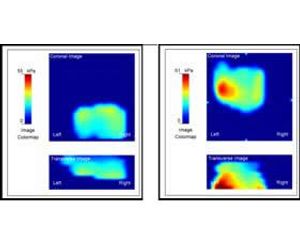
Using a sophisticated positioning system and complex mathematical algorithms, the ProUroScan technology is able to assemble the individual images it generates into a composite image of the prostate. Tissue exhibiting comparatively less elastic properties is identified by darker colors on the image, similar to how areas of precipitation are displayed on a weather radar map. The differences in elasticity that are identified and their position within the prostate help the physician to better characterize the patient’s status.
We believe the ProUroScan System’s existing technology provides a platform on which to develop multiple future generation systems. In the future, we intend to work to develop and introduce enhanced versions and additional indications for this technology. For example, this system may be able to monitor changes in prostate tissue over time, guide prostate biopsies, do prostate disease screening and assess changes in prostate size following drug treatment for benign prostate hyperplasia (“BPH”). Future generation systems will require us to obtain regulatory approval or clearance by conducting studies and filing additional submissions with the FDA.
Mechanical imaging is different from trans-rectal ultrasound (TRUS). The TRUS procedure serves as today’s gold standard for performing prostate biopsy because of its ability to see and guide the biopsy needles. It is not used as a basic screening or detection tool because of inadequate image quality. Other technologies like Magnetic Resonance Imaging (MRI) and Computerized Axial Tomography (CAT) scan or x-ray are also not used for basic prostate screening or detection. These later two technologies are also expensive and access to them outside of major medical centers is limited.
Our initial product is the ProUroScan system, developed and designed as a unique diagnostic-imaging system that enables physicians to accurately display and chart a prostate examination and identify prostate tissue abnormalities. Screening for abnormalities is currently accomplished by means of a digital rectal examination (“DRE”). A DRE is a qualitative and subjective test in which a physician wearing a latex glove inserts a lubricated finger into the rectal channel to feel or palpate the prostate gland for abnormalities. The clinician must rely on his or her experience and the sensitivity of their finger to estimate the size of the prostate and detect hardness or abnormalities within the prostate that may indicate the presence of potentially life-threatening disease. The ProUroScan is designed to produce a digital image of the prostate showing the size and symmetry of the prostate and the location of soft-tissue abnormalities within the prostate. We believe that the ProUroScan will enable a physician to detect abnormalities in the prostate more accurately than with their finger, assess areas of the prostate that the physician cannot reach with their finger, and produce quantifiable and chartable results.
The ProUroScan imaging system is being designed to detect certain abnormalities that may be missed by other diagnostic techniques. These abnormalities include areas on the surface or within the prostate where the tissue has a different level of elasticity or stiffness when compared with the rest of the gland, which can be precursors to a more clearly defined hardened area or nodule. The ProUroScan offers the advantage of being able to create an electronic record that can be stored within the system, printed and used for comparisons with new images created at a later date. No such electronic records or color images are possible when performing a traditional DRE.
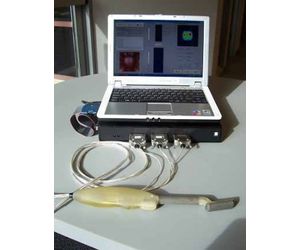
The ProUroScan system consists of a probe, a central processing unit containing proprietary image construction algorithms, a computer with color monitor and keyboard, a color printer, and single-use disposable sheaths to cover the tip of the probe. The ProUroScan probe is specially designed for the rectal anatomy to minimize discomfort. It is ergonomic for the clinician and similar to a traditional DRE for the patient. The probe utilizes pressure sensors located on the face of the probe head to palpate the prostate. The probe’s positioning system ensures that the person administering the test examines the entire surface of the prostate, and assists prostate image construction.
The probe attaches to the central processing unit which converts the information from the probe sensors with proprietary algorithms and then translates the data into a multicolor image on the monitor.
To perform a test, the clinician must first place a single-use disposable sheath over the probe head and shaft and cover it with a lubricant. The tip of the probe is then inserted into the patient’s rectum and palpates the prostate. As the prostate is palpated, a color image of the prostate is produced and displayed on the computer monitor, along with indicators of the amount of pressure being applied to help guide the clinician. Differences in tissue stiffness and elasticity will be depicted on the monitor in different colors. Total testing time for a healthy prostate should be under one minute.
Ideally, the ProUroScan will be administered by a trained clinician on all patients in need of a prostate exam. Standardizing the procedure using the ProUroScan mechanical imaging device should minimize the differences in abilities between physicians in this otherwise subjective procedure. We expect the system will also be more sensitive and have the ability to detect abnormalities that the normal human finger cannot detect, thereby improving the quality of the diagnostic process. It will also enable physicians to literally see the differences in tissue elasticity or stiffness on the color monitor instead of a traditional DRE that is “blind” and relies exclusively on the clinician’s sense of touch. The system will create a hard copy of the prostate diagnostic procedure which can be placed into the patient’s file. This will be advantageous for the physician on multiple fronts, as it gives the physician the ability to compare and contrast the patient’s results from exam to exam, and to get second opinions on the patient’s status in regards to the diagnosis.
We believe the ProUroScan is truly unique in the market. The closest comparison can be made to an examination using a transrectal ultrasound device. Transrectal ultrasound (“TRUS”) is a test that uses a rectal probe and sound wave echoes to create an image of the prostate gland, thus allowing visual inspection for abnormal conditions. Although well established as a highly accurate test, TRUS is used primarily as a guide for prostate biopsies, and is seldom used as a screening test. It is a very expensive test, costing hundreds of dollars each, difficult to interpret, and requires approximately 20 to 40 minutes to perform. The ProUroScan uses mechanical imaging rather than sound waves echoes, has a probe that is smaller than that of an ultrasound probe, will be performed in less than one minute, and at a much lower cost. The results of the test are displayed in color, making the results of the diagnostic test easy to interpret.
We anticipate that the majority of its revenue will be generated by usage fees based on consumption of proprietary disposable sheaths and/or probe tips. The systems themselves likely will be placed in clinics under a variety of programs, including operating leases, financing leases, outright sale, or placements paid for by premiums paid on the usage of the system.
We intend to position this product as an adjunctive test to DRE and Prostate Specific Antigen (“PSA”) blood tests, which have low sensitivity and specificity and cannot be documented with color images. The ProUroScan will be marketed towards primary care physicians and urologists. We believe the ProUroScan will help ensure that primary care physicians screen more effectively and quickly refer appropriate patients to urologists for more definitive testing.