

- Home
- Companies
- Thermo Fisher Scientific, LIMS & ...
- Products
- SpeciMAX - Saliva Collection Kit

SpeciMAX - Saliva Collection Kit
SpeciMAX Saliva Collection Kit is optimized to easily collect and transport saliva samples for SARS-CoV-2. Improve your high throughput SARS-CoV-2 surveillance testing with the SpeciMax Saliva Collection Kit. Your lab’s solution for efficient, cost-effective, standardized saliva collection compatible with downstream analysis workflows and automation systems.
Saliva collection kit optimized for your automated workflow
- Optimized for viral RNA extraction and direct to PCR workflows using Applied Biosystems MagMAX Viral/Pathogen kits (RUO),Thermo Scientific KingFisher Purification Systems,Applied Biosystems TaqCheck SARS-CoV-2 Fast PCR Assay (RUO), and TaqMan Applied Biosystems TaqMan® Assays (RUO)
- Standardized collection tubes sized to fit most automation sample racks with supplied barcodes
Space and cost savings compared to other collection kits
- No manual sample transfer step needed when used with automatic liquid handlers (see the time savings)
- Uses less 4°C refrigeration space than other collectors (Need room temperature storage? Request more information)
- No sample swabs to work around
Easy-to-use solution with non-invasive self-collection capability
- Secures 1-2 mL saliva in 6 mL polypropylene transfer tube
- Self-collection preferred by most users
Secure and trusted transport of saliva samples
- 95kPa compliant saliva collection tube for safe transport
- Single-use individually-packaged kits available in cases of 400 from a trusted single-source supplier (Looking for bulk ordering? Request more information)
To see full instructions for use visit SpeciMAX Saliva Collection Kit product page
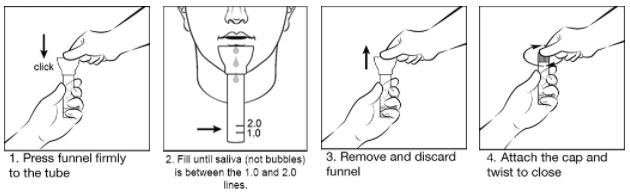

- Non-invasive sample collection
- Self-collected and less variability between users
- Easier laboratory workflow (no swabs to deal with)
- No mucus issues
- Preferred by most users

SpeciMAX Saliva Collection Kit fits into your direct to PCR or viral RNA extraction workflow. At Thermo Fisher Scientific, we offer both solutions depending on your needs.
SARS-CoV-2 surveillance testing for population-level occurrence

