

Lepu Medical Technology (Beijing) Co.,Ltd.
- Home
- Companies
- Lepu Medical Technology (Beijing) ...
- Products
- Lepu - Model NeoVas - Sirolimus-Eluting ...
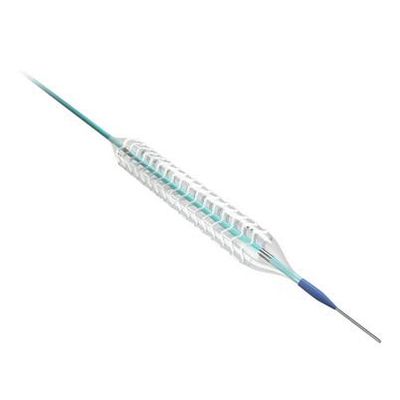
Lepu - Model NeoVas -Sirolimus-Eluting Bioresorbable Coronary Scaffold System
Stent Platform: Fully degradable poly(L-lactide). Sine wave structure in space, In-phase peak-valley connection.
Most popular related searches
balloon dilation catheter
stent system
drug coated
drug coating
muscle cell
cardiovascular product
dilatation catheter
medical device
medical device product
medical device production
Drug coating
- Fully degradable poly(D,L-lactide)
- Effectively control sirolimus release rate
Drug
- Classical Sirolimus with dose of 15.3ug/mm
- Inhibit the proliferation and migration of smooth muscle cells
Delivery system
- Completely self-developed second-generation rapid-exchange balloon dilatation catheter
- Superior pushability and crossability
NeoVas RCT Study
The study enrolled 560 subjects in 33 centers from China, Test group(NeoVas): 278 cases, Control group (Xience stent): 282 cases.
Conclusion:
The clinical events rate for Neovas bioabsorbable stent is low, showed equal safety and effectiveness with Xience metal stent.
NeoVas OPC study
The study enrolled 1103 subjects in 45 centers from China, two year clinical follow-up study has been completed to date.
