- Home
- Companies
- Vitro Biopharma
- Products
- AlloRx - Stem Cells
AlloRx - Stem Cells
AlloRx® Stem Cells is an investigational drug derived from the Wharton`s Jelly of the umbilical cord. Strict screening of the donor and medical/social history is evaluated prior to accepting cords. The cords are processed in Vitro Biopharma`s ISO 7 cleanroom with strict standard operating procedures and environmental controls. Once AlloRx® Stem Cells are produced, Vitro Biopharma goes through strict quality control testing to ensure the most sterile, safe, and potent stem cells. AlloRx® Stem Cells have been used in Institutional Review Board (IRB) approved clinical trials in international countries. Vitro Biopharma receives formal letters from the Administery of Health of these countries to ensure full compliance with both domestic and international regulations.
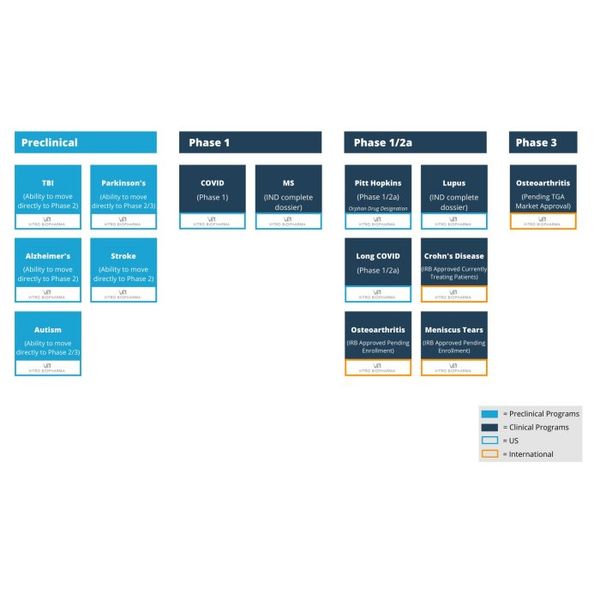
Vitro develops and commercializes adult stem cell technology for applications in stem cell research and drug development for the treatment of a vast variety of diseases and conditions. The company’s innovative and proprietary technology platform leverage umbilical cord derived stem cells, AlloRX® Stem Cells, translating ground-breaking science into medicines that transform the lives of patients with various disease. The cells when stored properly have an indefinite shelf life and are young, providing the most potent, vibrant stem cells in contrast to autologous. The current stem cell pipeline can be used to treat a multitude of diseases and is advancing multiple clinical trials across its product portfolio.