

- Home
- Companies
- OPIA Technologies
- Products
- EYEPRIM - Sterile Medical Device ...
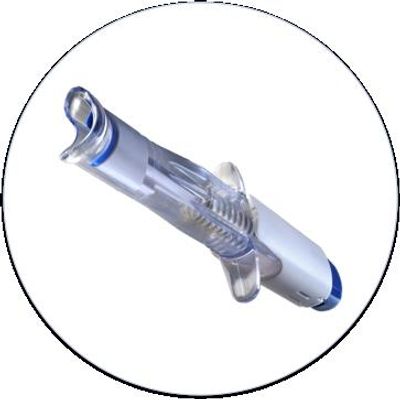
EYEPRIM - Sterile Medical Device Designed To Perform Conjunctival Impressions
EYEPRIM is a sterile medical device designed to perform conjunctival impressions of the living eye. It enables further analysis of cells and biomarkers to help diagnose several ocular surface disorders, such as dry eye, allergies or infections. The EYEPRIM device is based on an original idea, suggested by Christophe Baudouin, MD, PhD, Chairman of Department of Ophthalmology III at Quinze-Vingts Hospital and Research team leader at the Vision Institute, Team S12, Inserm-UPMC Research Center 968.
Why use EYEPRIM for conjunctival impressions?
The conjunctival impression technique allows the collection of cells from the conjunctiva in a nearly painless and non-invasive way, for the purpose of analyses (cytology, cytometry, PCR...) and diagnosis.
This technique is recommended by the TFOS (Tear Film & Ocular Surface Society) in the 2007 Report of the International Dry Eye Workshop.
EYEPRIM greatly facilitates the conjunctival impression technique, thus making it available for all practitioners. It is very much appreciated for the clinical studies follow-up thanks to its sampling reliability, high yield and very little training requirement. It is used in several clinical studies in US and Europe.
EYEPRIM is a Class I (sterile) medical device according to the directive 93/42/CEE, CE 0051 (IMQ) and FDA exempted from Premarket Authorization Procedure according to Code of Federal regulation Title 21: 21CFR880.6025.
