

NeuroStar - Model TMS -Therapy System
NeuroStar TMS Therapy is safe and easy to tolerate. Because it is not a drug that circulates throughout the body, NeuroStar TMS Therapy does not cause the side effects that patients often experience from antidepressant medications. NeuroStar TMS Therapy is free of systemic side effects such as5,6:
Common Side Effects from Drug Therapy*
- Abnormal ejaculation Impotence
- Decreased libido Constipation
- Diarrhea Nausea
- Daytime drowsiness Insomnia
- Nervousness Anxiety
- Increased weight Increased appetite
- Decreased appetite Weakness
- Dry mouth Dizziness
- Fatigue Headache/migraine
- Sweating Tremor
- Treatment discontinuation side effects
NeuroStar TMS Therapy Side Effects
In clinical trials, fewer than 5% of patients discontinued treatment with NeuroStar TMS Therapy due to adverse events. The most common side effect associated with NeuroStar TMS Therapy is temporary pain or discomfort at or near the treatment site during treatment. When this occurs it is temporary, and typically occurs only during the first week of treatment. Other side effects include eye pain, toothache, muscle twitch, facial pain and pain of the skin (occurring in ≥5% of patients and at 2 times the rate of placebo). There is a rare risk of seizure with TMS therapy (0.1% of patients).1,5
Patients should notify their doctor if they experience worsening depression symptoms, signs or symptoms of suicidal behavior and/or unusual behavior. Family members and support individuals should also be aware of the need to observe patients and notify their treatment provider if symptoms worsen.1
NeuroStar TMS Therapy should not be used with patients who have non-removable conductive metal or stimulator implants in or near the head.1
Transcranial magnetic stimulation, or TMS, uses a highly targeted pulsed magnetic field, similar in type and strength to those produced by a magnetic resonance imaging (MRI) machine, to stimulate cortical neurons.
- Precise pulsed magnetic fields induce small electric currents in the prefrontal cortex of the brain that activates deeper brain regions
- Local neurons depolarize, which leads to activation of deep brain structures via transsynaptic pathways
- Activation of these pathways in the limbic system leads to the release of neurotransmitters
- Blood flow and glucose metabolism rise in the activated regions, which is thought to result in improved mood
Neuroimaging studies have documented changes in cortical metabolic activity in tissue directly stimulated by TMS and in distal networks known to be involved in mood regulation.14
Repeated activation of the left prefrontal cortex is known to produce antidepressant effects in patients suffering from major depression.13
NeuroStar TMS Therapy provides targeted stimulation of the brain regions involved in mood regulation without the burden of systemic side effects.
NeuroStar TMS Therapy® is safe and effective, and can be conducted right in your doctor’s office.
- Each session takes 37 minutes
- Sessions are 5 days a week, for 4 to 6 weeks
During NeuroStar TMS Therapy, patients are awake and alert and can speak with the clinical operator if necessary. After treatment, patients can immediately return to normal activities. There are no effects on alertness or understanding; patients being treated with NeuroStar TMS Therapy can drive themselves to and from their treatment sessions.
Click on the video below to learn how NeuroStar TMS Therapy has helped reduce symptoms in people living with depression.
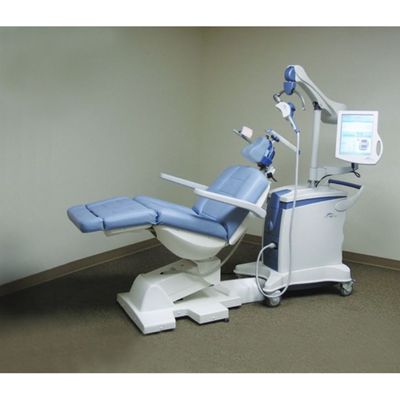
During treatment sessions, patients recline comfortably and relax in the treatment chair. A small curved device containing the magnetic coil rests lightly on the patient’s head and delivers focused magnetic stimulation directly to the areas of the brain that are underactive in patients with depression. The patient will hear a clicking sound and feel a tapping sensation on the head.
The most common side effect is generally mild-to-moderate pain or discomfort at or near the treatment area during the session. When this occurs it is temporary, and typically occurs only during the first week of treatment.1
Learn more about the established safety record of NeuroStar TMS Therapy.
NeuroStar TMS Therapy may not be right for everyone, so talk to a NeuroStar physician to learn more about how this proven depression treatment has helped other people with their depression, and whether it may help you or your loved one.
