

- Home
- Companies
- Pakumed Medical Products Gmbh
- Products
- Titan-Port Standard S
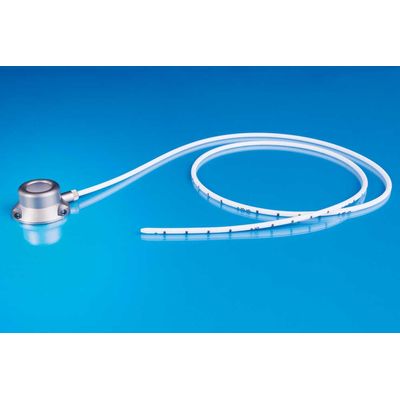
Titan-Port Standard S
TITAN-PORTS are totally implantable port catheter systems for venous implantation. They consist out of a titan chamber (port), a screw closure mechanism, a self-sealing silicone membrane and a catheter. Each system includes a 20 G access needle, a rinsing needle, a vein lifter, the instruction for use and an implant card. If the port catheter system is a complete set (recognisable by the suffix “SET” at the end of the article number), it also includes a special puncture cannula SFN 0925 S, a tunnelizer and a matching introducer set (e.g., for implantation using the Seldinger technique). The port catheter systems are placed on market as a procedure pack with difference in size of the port chamber, material and size of the catheter and a matching introducer set. The choice of size depends on the patient’s anatomy, the type of application, etc., and is at the discretion of the doctor.
TITAN port catheter systems ensure repeated access to the central venous vascular system for the following applications, e.g.:
- for long-term treatment of cytostatics agents and other “aggressive” medications
- patients with poor peripheral veins requiring frequent intravenous injections
- for infusion therapy
- for parenteral nutrition
- for HIV patients
- for venous blood sampling or blood transfusions
The advantage is a low risk of infection, simplified access and considerably improved quality of life for the patient as provided by a closed system.
Intended purpose: The port catheter system is only used to pass blood and medication into or out of the patient’s vascular system. The product itself thus fulfils a physical property and has no medical, therapeutic effect.
Patient group: Patients in need of long-term central venous access to the vascular system.
Operators: Medical professionals (doctors, nurses) with appropriate qualifications.
Only special non-coring port access needles (e.g., SFN® port needles or other suitable port needles) should be used to puncture the port membrane. These needles exhibit a unique bevel at the tip. 20 Gauge is recommended for standard use.
The use of special port needle prevents that holes or silicone particle are punched out, which could proceed into the bloodstream or plug the system.
